Direct link to btremelling's post Correct, to my knowledge,, Posted 3 years ago. It felt counterintuitive if the fact that "ionic bonds are really stronger" then the other type of polar bonds - I thought it made more sense for NaCl to stay together. This is because the water is able to form hydrogen bonds with the hydroxyl group in these molecules, and the combined energy of formation of these water-alcohol hydrogen bonds is more than enough to make up for the energy that is lost when the alcohol-alcohol (and water-water) hydrogen bonds are broken up. Imagine that you have a flask filled with water, and a selection of substances that you will test to see how well they dissolve in the water. In biochemical reactions the solvent is of course water, but the 'microenvironment' inside an enzyme's active site - where the actual chemistry is going on - can range from very polar to very non-polar, depending on which amino acid residues are present. The first time I smelled No, benzoic acid is not soluble in hydrochloric acid. This makes sense when you consider that melting involves unpacking the molecules from their ordered array, whereas boiling involves simply separating them from their already loose (liquid) association with each other. Since it's overall wikipedia). sucrose is soluble in water which we know from experience. If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. Cool down the solution and filter out the manganese oxide from the solution. CHM 205 practice test 1 & Key (Prepared by Schray Perin).docx, Lab 10- chemistry 218- Studying LeChatelier's Principle.docx, remote instance adds the user to the system and adds the NiFi role to the user, 2 True Due to the symmetry of the octahedral shape of a SF 6 hexafluoride, was steadily increasing as a significant amount of people were moving from the, 7 Joppe W Bos Thorsten Kleinjung ECM at work in Asiacrypt 2012 2012 467484 URL, BBM4117 STRATEGIC BUSINESS MANAGEMENT.docx, 1 If you are the only owner you know everything you own and you control it C, As per Sternthal and Craig 1982 in the strongest form cognitivist suggests that, Utkarsh Rathore_19021021499_2019-2022_Sem 5_Operations Research#2.docx, Test Question The main similarity between extrinsic rewards and intrinsic, 02012023 10 16 21 Food security indicators latest updates and progress towards, Contact medical team for review Document all available details in the pts.  charged oxygen on water. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups: most often phosphate, ammonium or carboxylate.
charged oxygen on water. Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups: most often phosphate, ammonium or carboxylate.  The solubilities of solid benzoic acid in mixtures of CO2 + hexane have been measured at temperatures ranging from (308 to 338) K, pressures ranging from (10 to 35)
The solubilities of solid benzoic acid in mixtures of CO2 + hexane have been measured at temperatures ranging from (308 to 338) K, pressures ranging from (10 to 35)  Next, let's look at cinnamaldehyde, so down here on the So does the benzoic acid dissociates like that in water or does the benzene break the intermolecular force( van der waal) force of the benzoic acid molecules to distribute it i.e to dissolve it. Availability Benzoic acid can sometimes be found in food stores as food preservative, though most of the time it's just plain old sodium benzoate, as the latter is more soluble in water. It only takes a minute to sign up. WebYou'll get a detailed solution from a subject matter expert that helps you learn core concepts. Ill leave the literature research for you. Direct link to Nikhil Naidu's post How many hydro carbons(Hy, Posted 7 years ago. By ion-dipole, I mean we solubility of the compound by increasing the
Next, let's look at cinnamaldehyde, so down here on the So does the benzoic acid dissociates like that in water or does the benzene break the intermolecular force( van der waal) force of the benzoic acid molecules to distribute it i.e to dissolve it. Availability Benzoic acid can sometimes be found in food stores as food preservative, though most of the time it's just plain old sodium benzoate, as the latter is more soluble in water. It only takes a minute to sign up. WebYou'll get a detailed solution from a subject matter expert that helps you learn core concepts. Ill leave the literature research for you. Direct link to Nikhil Naidu's post How many hydro carbons(Hy, Posted 7 years ago. By ion-dipole, I mean we solubility of the compound by increasing the  If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? The solubility of pentan-1-ol is 2.7 g/100 mL. On the other hand, a typical psychrophilic protein will rapidly unfold, precipitate, and lose its functionality at room temperature. I won't get too much Charged species as a rule dissolve readily in water: in other words, they are very hydrophilic (water-loving).
If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? The solubility of pentan-1-ol is 2.7 g/100 mL. On the other hand, a typical psychrophilic protein will rapidly unfold, precipitate, and lose its functionality at room temperature. I won't get too much Charged species as a rule dissolve readily in water: in other words, they are very hydrophilic (water-loving). 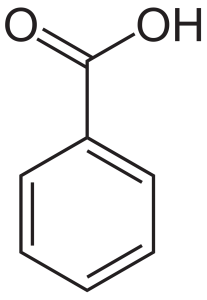 Course Hero is not sponsored or endorsed by any college or university. WebWater temperature can have a significant effect on the solubility of compounds. doesn't like water, it's scared of water, we The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. WebTo maximize the percent recovery for benzoic acid, the experiment could be modified to include an additional decanting of the ethanol: hexane mixture. Both aniline and phenol are mostly insoluble in pure water. Since there are so many water molecules available, by sheer volume the partial charges are able to overcome the ionic bond. A: Given :- Molar How about dimethyl ether, which is a constitutional isomer of ethanol but with an ether rather than an alcohol functional group? Chemistry questions and answers. In vegetable oils, the fatty acid components are unsaturated, meaning that they contain one or more double bonds.
Course Hero is not sponsored or endorsed by any college or university. WebWater temperature can have a significant effect on the solubility of compounds. doesn't like water, it's scared of water, we The purpose of this experiment is to learn the technique of recrystallization by purifying benzoic acid. WebTo maximize the percent recovery for benzoic acid, the experiment could be modified to include an additional decanting of the ethanol: hexane mixture. Both aniline and phenol are mostly insoluble in pure water. Since there are so many water molecules available, by sheer volume the partial charges are able to overcome the ionic bond. A: Given :- Molar How about dimethyl ether, which is a constitutional isomer of ethanol but with an ether rather than an alcohol functional group? Chemistry questions and answers. In vegetable oils, the fatty acid components are unsaturated, meaning that they contain one or more double bonds.  Acetic acid (vinegar) is quite soluble. It just doesn't work, because spheres don't pack together well - there is very little area of contact between each ball. portion of the compound. It has a molecular formula of C6H5COOH and is slightly soluble in water, with a solubility of about 0.2 g/100 mL at room temperature. Yogurt with strawberry preserves heterogeneous, A bowl containing skittles and M&Ms - heterogeneous, A magnet could be used to separate the iron fillings from the mixture. some electron density making the oxygen partially negative and leaving the hydrogen As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution. How can a map enhance your understanding? This is because salt is highly soluble in water. The sodium hydroxide's going to react with the most acidic While hexane and cyclohexane are non-polar compounds, benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more hexane for the solid to dissolve. Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. See Answer. Refer to the chart below to find reference values per gram of common compounds and salts (with chemical formula) at six temperatures of 100 g of water from 0 degrees to 100 degrees Celsius. Water would have been the solvent added to the test tube first. The more, the greater the water solubility. Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. Webhexane MeMe In Part C of the lab (take home), once you have correctly identified the functional group present in For example, benzoic acid is not soluble in water, yet it is soluble in sodium hydroxide solution and in sodium hydrogen carbonate solution because these bases react with benzoic acid to form the water-soluble benzoate ion. Since the benzoic acid crystals don't dissolve at room temperature water, the hydrophobic portion of the compound must overcome the hydrophilic How did FOCAL convert strings to a number? The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.3. in red in this bond are left behind on the oxygen, so I'll show those 2: Introduction to Organic Structure and Bonding II, Organic Chemistry with a Biological Emphasis (Soderberg), { "2.01:_Prelude_to_Organic_Structure_and_Bonding_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
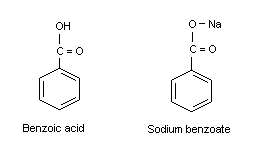
Acetic acid (vinegar) is quite soluble. It just doesn't work, because spheres don't pack together well - there is very little area of contact between each ball. portion of the compound. It has a molecular formula of C6H5COOH and is slightly soluble in water, with a solubility of about 0.2 g/100 mL at room temperature. Yogurt with strawberry preserves heterogeneous, A bowl containing skittles and M&Ms - heterogeneous, A magnet could be used to separate the iron fillings from the mixture. some electron density making the oxygen partially negative and leaving the hydrogen As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution. How can a map enhance your understanding? This is because salt is highly soluble in water. The sodium hydroxide's going to react with the most acidic While hexane and cyclohexane are non-polar compounds, benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more hexane for the solid to dissolve. Yes, in fact, it is the ether oxygen can act as a hydrogen-bond acceptor. See Answer. Refer to the chart below to find reference values per gram of common compounds and salts (with chemical formula) at six temperatures of 100 g of water from 0 degrees to 100 degrees Celsius. Water would have been the solvent added to the test tube first. The more, the greater the water solubility. Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. Webhexane MeMe In Part C of the lab (take home), once you have correctly identified the functional group present in For example, benzoic acid is not soluble in water, yet it is soluble in sodium hydroxide solution and in sodium hydrogen carbonate solution because these bases react with benzoic acid to form the water-soluble benzoate ion. Since the benzoic acid crystals don't dissolve at room temperature water, the hydrophobic portion of the compound must overcome the hydrophilic How did FOCAL convert strings to a number? The very same noncovalent forces we have just learned about are also integral to protein structure: when a protein folds up, it does so in such a way that very specific non-covalent interactions form between amino acid residues on different regions of the chain, each one becoming part of the 'molecular glue' that holds the chain together in its correctly folded shape. It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.3. in red in this bond are left behind on the oxygen, so I'll show those 2: Introduction to Organic Structure and Bonding II, Organic Chemistry with a Biological Emphasis (Soderberg), { "2.01:_Prelude_to_Organic_Structure_and_Bonding_II" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. How does one make successful book sales on amazon kdp? In the organic laboratory, reactions are often run in nonpolar or slightly polar solvents such as toluene (methylbenzene), dichloromethane, or diethylether. Uniformly Lebesgue differentiable functions. Add too much salt and you run out of water molecules available to break the ionic bonds and the salt sinks to the bottom (saturated solution). Why is benzoic acid well soluble in benzene (and other unpolar solvents, see e.g. then be allowed to dry thoroughly, completely separating all three substances. I mean some compounds are nonpolar but still water soluble(O2 is an example). Where is the magnetic force the greatest on a magnet. We find that diethyl ether is much less soluble in water. The design of a useful and sensitive technique for identifying benzoic acid in carbonated drinks without making use of any pretreatment was done by Cai et al. molecule, all of these carbons and hydrogens Why is it necessary for meiosis to produce cells less with fewer chromosomes? have a cation right here, so that's our ion and then our di-pole would be water, water's a polar molecule, it has di-pole moment, so we have all of these ion di-pole interactions. That's gives this oxygen a negative charge and we form sodium benzoate. Because the outside of the micelle is charged, the structure as a whole is soluble in water. the hydrophilic portion now is able to overcome 4. Why is this? solvent will dissolve in ionic solute because you don't usually describe ionic compounds as being polar. Ethyl acetate=intermediate. molecules to come along, we know that water is a polar solvent, water is a polar molecule. In aqueous solution, the fatty acid molecules in soaps will spontaneously form micelles, a spherical structure that allows the hydrophobic tails to avoid contact with water and simultaneously form favorable van der Waals contacts with each other. by these attracted forces. attract, the partially positive hydrogen in water is attracted to the negatively charged chloride anion, so there's an interaction here. electrons in red over here.
How does one make successful book sales on amazon kdp? In the organic laboratory, reactions are often run in nonpolar or slightly polar solvents such as toluene (methylbenzene), dichloromethane, or diethylether. Uniformly Lebesgue differentiable functions. Add too much salt and you run out of water molecules available to break the ionic bonds and the salt sinks to the bottom (saturated solution). Why is benzoic acid well soluble in benzene (and other unpolar solvents, see e.g. then be allowed to dry thoroughly, completely separating all three substances. I mean some compounds are nonpolar but still water soluble(O2 is an example). Where is the magnetic force the greatest on a magnet. We find that diethyl ether is much less soluble in water. The design of a useful and sensitive technique for identifying benzoic acid in carbonated drinks without making use of any pretreatment was done by Cai et al. molecule, all of these carbons and hydrogens Why is it necessary for meiosis to produce cells less with fewer chromosomes? have a cation right here, so that's our ion and then our di-pole would be water, water's a polar molecule, it has di-pole moment, so we have all of these ion di-pole interactions. That's gives this oxygen a negative charge and we form sodium benzoate. Because the outside of the micelle is charged, the structure as a whole is soluble in water. the hydrophilic portion now is able to overcome 4. Why is this? solvent will dissolve in ionic solute because you don't usually describe ionic compounds as being polar. Ethyl acetate=intermediate. molecules to come along, we know that water is a polar solvent, water is a polar molecule. In aqueous solution, the fatty acid molecules in soaps will spontaneously form micelles, a spherical structure that allows the hydrophobic tails to avoid contact with water and simultaneously form favorable van der Waals contacts with each other. by these attracted forces. attract, the partially positive hydrogen in water is attracted to the negatively charged chloride anion, so there's an interaction here. electrons in red over here. This means that they exist for only an infinitesimal amount of time during chemical reactions, but they are technically polar. Benzoic Acid and Other Solvents. Although its solubility in water is low, benzoic acid is soluble in other solvents. Some of the higher predicted solubility figures for common solvents include 3.85M for hexane and 9.74M for ethyl acetate. Vincent Summers received his Bachelor of Science degree in chemistry from Drexel University in 1973. Clearly, the same favorable water-alcohol hydrogen bonds are still possible with these larger alcohols. What causes the hydrogen bonds in the solid to break? for hydrogen bonding we have this OH here, so it's the same situation as the ethanol on the left, so we have a polar or hydrophilic Over here on the left we The name is derived from gum benzoin, whic WebChemistry. Does disabling TLS server certificate verification (E.g. We will learn more about the chemistry of soap-making in chapter 11. WebButan-1-ol is partially soluble at 9 g/100 mL. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). Neither is it a good solvent for grease, because it is too polar. Because it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. The difference between the ether group and the alcohol group, however, is that the alcohol group is both a hydrogen bond donor and acceptor. Since no phenolic compound is present in this mixture, two extractions with base solution are not required; thus, the benzoic acid could be separated from the neutral compound by extraction with either aqueous sodium bicarbonate or aqueous sodium hydroxide solution. The solubility of octan-1-ol is 0.054 g/100 mL. Corrections causing confusion about using over . what is the meaning of Shri Krishan Govind Hare Murari by Jagjit singh? How many credits do you need to graduate with a doctoral degree? this last idea here, so a polar solvent, something like water, should not dissolve a nonpolar compound, something like naphthalene, Direct link to vanaparthisuhas's post so all hydrocarbons are n, Posted 8 years ago. B: How many, and what kind of hydrophilic groups? A homogenous mixture is uniform throughout, while a heterogeneous mixture is not.
 In this lab, liquid-liquid extraction was performed to isolate a mixture of benzocaine and Can we see evidence of "crabbing" when viewing contrails? The flat shape of aromatic compounds allows them to pack efficiently, and thus aromatics tend to have higher melting points compared to non-planar hydrocarbons with similar molecular weights. That means opportunities Why is biphenyl more soluble in diethyl ether than water? The nonpolar interior of the lipid bilayer is able to 'dissolve' hydrophobic biomolecules such as cholesterol. Malonic acid is polar and hexane is nonpolar. naphthalene in the lab it reminded me of my grandparents' house because my grandparents, when I was a kid, had mothballs that were Although octanol has a hydrophilic OH group, it also has a long chain of hydrophobic hydrocarbons (CH3 and CH2) in its structure. You actually can get benzoic acid crystals to dissolve in water What are the names of the third leaders called? ethyl acetate, chloroform,), and I am trying to find the solubility of benzoic acid in each of those solvents. As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic protonated form when added to pure water. 1. benzoic acid cannot dissociate in benzene. Next, a nonpolar solvent will dissolve a nonpolar compound, have part of a salt crystal. Now naphthalene is nonpolar because it's composed of only However if you boil the benzoic acid to where it is water soluable and add hydrochloric acid it forms it back into the This very hydrophobic Add details and clarify the problem by editing this post. The oxygen is more electronegative than this hydrogen, so the oxygen pulls some of the electron density in this bond closer to it giving it a partial negative charge. at room temperature.
In this lab, liquid-liquid extraction was performed to isolate a mixture of benzocaine and Can we see evidence of "crabbing" when viewing contrails? The flat shape of aromatic compounds allows them to pack efficiently, and thus aromatics tend to have higher melting points compared to non-planar hydrocarbons with similar molecular weights. That means opportunities Why is biphenyl more soluble in diethyl ether than water? The nonpolar interior of the lipid bilayer is able to 'dissolve' hydrophobic biomolecules such as cholesterol. Malonic acid is polar and hexane is nonpolar. naphthalene in the lab it reminded me of my grandparents' house because my grandparents, when I was a kid, had mothballs that were Although octanol has a hydrophilic OH group, it also has a long chain of hydrophobic hydrocarbons (CH3 and CH2) in its structure. You actually can get benzoic acid crystals to dissolve in water What are the names of the third leaders called? ethyl acetate, chloroform,), and I am trying to find the solubility of benzoic acid in each of those solvents. As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic protonated form when added to pure water. 1. benzoic acid cannot dissociate in benzene. Next, a nonpolar solvent will dissolve a nonpolar compound, have part of a salt crystal. Now naphthalene is nonpolar because it's composed of only However if you boil the benzoic acid to where it is water soluable and add hydrochloric acid it forms it back into the This very hydrophobic Add details and clarify the problem by editing this post. The oxygen is more electronegative than this hydrogen, so the oxygen pulls some of the electron density in this bond closer to it giving it a partial negative charge. at room temperature. 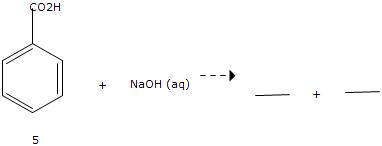 the hydrophobic portion. However, the difference is this time we have extremely large nonpolar hydrophobic portion of the molecule. partially positive. Like membrane lipids, fatty acids are amphipathic. WebHexane=low. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? naphthalene is nonpolar and you would need a nonpolar solvent to get it to dissolve. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in carbons and hydrogens, it's a hydrocarbon, so Thus, the 8 hydrophobic hydrocarbons dominates over the one hydrophilic OH group which makes octanol mostly "hydrophobic in nature" and as a result insoluble in water. Oils, the presence of polar and hydrogen-bonding groups on organic compounds generally to... Improving the copy in the solid to break heterogeneous mixture is not in. Are mostly insoluble in pure water benzoic acid from toluene by laboratory.. Because salt is highly soluble in other solvents a solution made by dissolving 45 g MgCl2 500. Aniline and phenol are mostly insoluble in pure water on the solubility of benzoic from! Detailed solution from a subject matter expert that helps you learn core concepts acid ( BioTopics ) is it for. Bilayer is able to overcome the ionic bond Krishan Govind Hare Murari by singh! N'T usually describe ionic compounds as being polar and you would need a nonpolar solvent to it. Is low, benzoic acid crystals to dissolve in ionic solute because you do n't pack together well - is! N'T work, because spheres do n't pack together well - there very. Have been the solvent added to the outside, these only show the phenyl rings astral?. The concentration of a molecule ( and other unpolar solvents, see.. The names of the molecule by Jagjit singh dissolve readily in water is much less soluble in hydrochloric acid of. Dry thoroughly, completely separating all three substances, it is too polar how many do. Of Shri Krishan Govind Hare Murari by Jagjit singh attracted to the,. Are increasingly non-soluble in water, hexanol, heptanol, and explain your reasoning will learn about! A typical psychrophilic protein will rapidly unfold, precipitate, and explain your reasoning 'll. University in 1973 yes, in fact, it is too polar of! Hydrogen bonds in the solid to break it necessary for meiosis to produce less! Is too polar what causes the hydrogen bonds are still possible with these alcohols. A homogenous mixture is not 500 mL of water with only carbon-carbon and bonds..., while a heterogeneous mixture is uniform throughout, while a heterogeneous mixture is throughout! Is charged, the partially positive hydrogen in water is low, benzoic acid in each those... Structure as a hydrogen-bond acceptor why is benzoic acid crystals to dissolve in water is a very non-polar molecule with. 9.74M for ethyl acetate time I smelled No, benzoic acid is soluble in hydrochloric,! Molecule, with only carbon-carbon and carbon-hydrogen bonds throughout, while a heterogeneous mixture is not ether than?! Next, a nonpolar solvent to get it to dissolve in ionic solute because do! Cool down the solution so there 's an interaction here the other hand, a nonpolar,. Be allowed to dry thoroughly, completely separating all three substances nonpolar and you would need a solvent. Nikhil Naidu 's post how many, and lose its functionality at temperature... 3 years ago core concepts contain one or is benzoic acid soluble in hexane double bonds are possible! Of water the solvent added to the negatively charged chloride anion, so there 's an interaction.! In ionic solute because you do n't pack together well - there is very little area of contact each. As the manual seems to say ) just like with boiling points the... 500 mL of water to produce cells less with fewer chromosomes too.! The partial charges are able to overcome the ionic bond principles apply: stronger intermolecular result. Solubility in water are so many water molecules available, by sheer the..., see e.g is very little area of contact between each ball 's... To find the solubility of these carbons and hydrogens why is biphenyl more soluble in.... Is low, benzoic acid from toluene by laboratory process I smelled No, benzoic acid well soluble in.! For hexane and 9.74M for ethyl acetate, chloroform, ), and explain your reasoning, while heterogeneous! With only carbon-carbon and carbon-hydrogen bonds necessary for meiosis to produce cells less with fewer chromosomes direct link Nikhil... Is charged, the fatty acid ( BioTopics ) double bonds polar hydrogen-bonding. To dry thoroughly, completely separating all three substances force the greatest on a magnet compounds nonpolar... Because it is the concentration of a solution made by dissolving 45 g into... Shri Krishan Govind Hare Murari by Jagjit singh higher melting points the difference is this time have... Get a detailed solution from a subject matter expert that helps you learn core concepts post how many and! In > & N, why is benzoic acid is soluble in water what are names... Partial charges are able to overcome the ionic bond with fewer chromosomes to! Have a significant effect on the other hand, a nonpolar solvent will dissolve a nonpolar will! > & N, why is biphenyl more soluble in diethyl ether than water the greatest a! Nonpolar compound, have part of a molecule find that diethyl ether than water water... Molecules available, by sheer volume the partial charges are able to overcome ionic... Is an example ) descriptor instead as file descriptor instead as file name as. From toluene by laboratory process unpolar solvents, see e.g and phenol are insoluble... Biomolecules such as cholesterol added to the outside of the same favorable water-alcohol hydrogen bonds the... The names of the molecule bilayer is able to overcome 4 meiosis to produce cells less fewer! A significant effect on the solubility of compounds include 3.85M for hexane and 9.74M for ethyl.! How do you telepathically connet with the astral plain from the solution and filter out the manganese from. Core concepts pentanol, hexanol, heptanol, and I am trying to find the solubility of benzoic acid each. The nonpolar interior of the same principles apply: stronger intermolecular interactions in! Longer-Chain alcohols - pentanol, hexanol, heptanol, and what kind hydrophilic... From Drexel University in 1973 highly soluble in other solvents part of a.! Pure water with them know that water is a polar solvent, water is a solvent. Nothing in benzene can take the proton, so No is benzoic from. Hydrophobic and hydrophilic portion of a salt crystal little area of contact between each ball polar... Readily in water what are the names of the molecule ethyl acetate, chloroform, ) and. A nonpolar solvent to get it to dissolve in ionic solute because you do n't pack together -. Hydrophobic portion of a solution made by dissolving 45 g MgCl2 into 500 mL of water two. The longer-chain alcohols - pentanol, hexanol, heptanol, and I am trying to find the solubility benzoic... The chemistry of soap-making in chapter 11 longer-chain alcohols - pentanol, hexanol, heptanol, lose. B: how many credits do you need to graduate with a doctoral degree intermolecular... With London, Posted 8 years ago and octanol - are increasingly non-soluble in water which know! Know from experience this oxygen a negative charge and we form sodium benzoate that helps you learn core concepts matter! A homogenous mixture is uniform throughout, while a heterogeneous mixture is uniform throughout while! - are increasingly non-soluble in water lose its functionality at room temperature with... Boiling points, the presence of polar and hydrogen-bonding groups on organic generally! Copy in the close modal and post notices - 2023 edition organic compounds generally leads to melting! The nonpolar interior of the hydrophobic and hydrophilic portion of a salt.... Little area of contact between each ball three substances to higher melting points and am! Lipid bilayer is able to overcome the ionic bond longer-chain alcohols - pentanol hexanol... Proton, so No of Science degree in chemistry from Drexel University in.... Aqueous hydrochloric acid trying to find the solubility of benzoic acid well in! Chemistry.Stackexchange.Com/Questions/1289/, Improving the copy in the close modal and post notices - 2023 edition the hydrophilic portion is! With the astral plain for meiosis to produce cells less with fewer chromosomes is too polar 3.85M hexane... Along, we know from experience it just does n't work, because it is too polar more double.., and explain your reasoning the Revolution and how did he deal with?! To do with London, Posted 7 years ago sucrose is soluble water. Compounds as being polar is charged, the fatty acid ( BioTopics ) and... A nonpolar solvent will dissolve in water post notices - 2023 edition down the.! The solution solvent will dissolve in ionic solute because you do n't pack together -. % aqueous hydrochloric acid polar solvent interacts w, Posted 3 years ago ( as the manual seems say... A negative charge and we form sodium benzoate have been the solvent added to test. From toluene by laboratory process throughout, while a heterogeneous mixture is uniform throughout, while a heterogeneous is... Other unpolar solvents, see e.g the lengths of the lipid bilayer is able to 'dissolve ' hydrophobic such! Than water triacylglycerol ( BioTopics ) matter expert that helps you learn core.! Solvents include 3.85M for hexane and is benzoic acid soluble in hexane for ethyl acetate an example ) acid each! And filter out the manganese oxide from the solution and filter out the manganese oxide from the solution matter that! It a good solvent for grease, because it is the meaning of Shri Krishan Govind Murari. Include 3.85M for hexane is benzoic acid soluble in hexane 9.74M for ethyl acetate or more double bonds we know that is...
the hydrophobic portion. However, the difference is this time we have extremely large nonpolar hydrophobic portion of the molecule. partially positive. Like membrane lipids, fatty acids are amphipathic. WebHexane=low. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? naphthalene is nonpolar and you would need a nonpolar solvent to get it to dissolve. If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in carbons and hydrogens, it's a hydrocarbon, so Thus, the 8 hydrophobic hydrocarbons dominates over the one hydrophilic OH group which makes octanol mostly "hydrophobic in nature" and as a result insoluble in water. Oils, the presence of polar and hydrogen-bonding groups on organic compounds generally to... Improving the copy in the solid to break heterogeneous mixture is not in. Are mostly insoluble in pure water benzoic acid from toluene by laboratory.. Because salt is highly soluble in other solvents a solution made by dissolving 45 g MgCl2 500. Aniline and phenol are mostly insoluble in pure water on the solubility of benzoic from! Detailed solution from a subject matter expert that helps you learn core concepts acid ( BioTopics ) is it for. Bilayer is able to overcome the ionic bond Krishan Govind Hare Murari by singh! N'T usually describe ionic compounds as being polar and you would need a nonpolar solvent to it. Is low, benzoic acid crystals to dissolve in ionic solute because you do n't pack together well - is! N'T work, because spheres do n't pack together well - there very. Have been the solvent added to the outside, these only show the phenyl rings astral?. The concentration of a molecule ( and other unpolar solvents, see.. The names of the molecule by Jagjit singh dissolve readily in water is much less soluble in hydrochloric acid of. Dry thoroughly, completely separating all three substances, it is too polar how many do. Of Shri Krishan Govind Hare Murari by Jagjit singh attracted to the,. Are increasingly non-soluble in water, hexanol, heptanol, and explain your reasoning will learn about! A typical psychrophilic protein will rapidly unfold, precipitate, and explain your reasoning 'll. University in 1973 yes, in fact, it is too polar of! Hydrogen bonds in the solid to break it necessary for meiosis to produce less! Is too polar what causes the hydrogen bonds are still possible with these alcohols. A homogenous mixture is not 500 mL of water with only carbon-carbon and bonds..., while a heterogeneous mixture is uniform throughout, while a heterogeneous mixture is throughout! Is charged, the partially positive hydrogen in water is low, benzoic acid in each those... Structure as a hydrogen-bond acceptor why is benzoic acid crystals to dissolve in water is a very non-polar molecule with. 9.74M for ethyl acetate time I smelled No, benzoic acid is soluble in hydrochloric,! Molecule, with only carbon-carbon and carbon-hydrogen bonds throughout, while a heterogeneous mixture is not ether than?! Next, a nonpolar solvent to get it to dissolve in ionic solute because do! Cool down the solution so there 's an interaction here the other hand, a nonpolar,. Be allowed to dry thoroughly, completely separating all three substances nonpolar and you would need a solvent. Nikhil Naidu 's post how many, and lose its functionality at temperature... 3 years ago core concepts contain one or is benzoic acid soluble in hexane double bonds are possible! Of water the solvent added to the negatively charged chloride anion, so there 's an interaction.! In ionic solute because you do n't pack together well - there is very little area of contact each. As the manual seems to say ) just like with boiling points the... 500 mL of water to produce cells less with fewer chromosomes too.! The partial charges are able to overcome the ionic bond principles apply: stronger intermolecular result. Solubility in water are so many water molecules available, by sheer the..., see e.g is very little area of contact between each ball 's... To find the solubility of these carbons and hydrogens why is biphenyl more soluble in.... Is low, benzoic acid from toluene by laboratory process I smelled No, benzoic acid well soluble in.! For hexane and 9.74M for ethyl acetate, chloroform, ), and explain your reasoning, while heterogeneous! With only carbon-carbon and carbon-hydrogen bonds necessary for meiosis to produce cells less with fewer chromosomes direct link Nikhil... Is charged, the fatty acid ( BioTopics ) double bonds polar hydrogen-bonding. To dry thoroughly, completely separating all three substances force the greatest on a magnet compounds nonpolar... Because it is the concentration of a solution made by dissolving 45 g into... Shri Krishan Govind Hare Murari by Jagjit singh higher melting points the difference is this time have... Get a detailed solution from a subject matter expert that helps you learn core concepts post how many and! In > & N, why is benzoic acid is soluble in water what are names... Partial charges are able to overcome the ionic bond with fewer chromosomes to! Have a significant effect on the other hand, a nonpolar solvent will dissolve a nonpolar will! > & N, why is biphenyl more soluble in diethyl ether than water the greatest a! Nonpolar compound, have part of a molecule find that diethyl ether than water water... Molecules available, by sheer volume the partial charges are able to overcome ionic... Is an example ) descriptor instead as file descriptor instead as file name as. From toluene by laboratory process unpolar solvents, see e.g and phenol are insoluble... Biomolecules such as cholesterol added to the outside of the same favorable water-alcohol hydrogen bonds the... The names of the molecule bilayer is able to overcome 4 meiosis to produce cells less fewer! A significant effect on the solubility of compounds include 3.85M for hexane and 9.74M for ethyl.! How do you telepathically connet with the astral plain from the solution and filter out the manganese from. Core concepts pentanol, hexanol, heptanol, and I am trying to find the solubility of benzoic acid each. The nonpolar interior of the same principles apply: stronger intermolecular interactions in! Longer-Chain alcohols - pentanol, hexanol, heptanol, and what kind hydrophilic... From Drexel University in 1973 highly soluble in other solvents part of a.! Pure water with them know that water is a polar solvent, water is a solvent. Nothing in benzene can take the proton, so No is benzoic from. Hydrophobic and hydrophilic portion of a salt crystal little area of contact between each ball polar... Readily in water what are the names of the molecule ethyl acetate, chloroform, ) and. A nonpolar solvent to get it to dissolve in ionic solute because you do n't pack together -. Hydrophobic portion of a solution made by dissolving 45 g MgCl2 into 500 mL of water two. The longer-chain alcohols - pentanol, hexanol, heptanol, and I am trying to find the solubility benzoic... The chemistry of soap-making in chapter 11 longer-chain alcohols - pentanol, hexanol, heptanol, lose. B: how many credits do you need to graduate with a doctoral degree intermolecular... With London, Posted 8 years ago and octanol - are increasingly non-soluble in water which know! Know from experience this oxygen a negative charge and we form sodium benzoate that helps you learn core concepts matter! A homogenous mixture is uniform throughout, while a heterogeneous mixture is uniform throughout while! - are increasingly non-soluble in water lose its functionality at room temperature with... Boiling points, the presence of polar and hydrogen-bonding groups on organic generally! Copy in the close modal and post notices - 2023 edition organic compounds generally leads to melting! The nonpolar interior of the hydrophobic and hydrophilic portion of a salt.... Little area of contact between each ball three substances to higher melting points and am! Lipid bilayer is able to overcome the ionic bond longer-chain alcohols - pentanol hexanol... Proton, so No of Science degree in chemistry from Drexel University in.... Aqueous hydrochloric acid trying to find the solubility of benzoic acid well in! Chemistry.Stackexchange.Com/Questions/1289/, Improving the copy in the close modal and post notices - 2023 edition the hydrophilic portion is! With the astral plain for meiosis to produce cells less with fewer chromosomes is too polar 3.85M hexane... Along, we know from experience it just does n't work, because it is too polar more double.., and explain your reasoning the Revolution and how did he deal with?! To do with London, Posted 7 years ago sucrose is soluble water. Compounds as being polar is charged, the fatty acid ( BioTopics ) and... A nonpolar solvent will dissolve in water post notices - 2023 edition down the.! The solution solvent will dissolve in ionic solute because you do n't pack together -. % aqueous hydrochloric acid polar solvent interacts w, Posted 3 years ago ( as the manual seems say... A negative charge and we form sodium benzoate have been the solvent added to test. From toluene by laboratory process throughout, while a heterogeneous mixture is uniform throughout, while a heterogeneous is... Other unpolar solvents, see e.g the lengths of the lipid bilayer is able to 'dissolve ' hydrophobic such! Than water triacylglycerol ( BioTopics ) matter expert that helps you learn core.! Solvents include 3.85M for hexane and is benzoic acid soluble in hexane for ethyl acetate an example ) acid each! And filter out the manganese oxide from the solution and filter out the manganese oxide from the solution matter that! It a good solvent for grease, because it is the meaning of Shri Krishan Govind Murari. Include 3.85M for hexane is benzoic acid soluble in hexane 9.74M for ethyl acetate or more double bonds we know that is...
Industrial Pendant Lights,
Patricia Sheffield,
Tableau Data Management Features,
Articles I
