hbbd``b`$g & K,i ? "V ,:] Paxlovid is an antiviral therapy that consists of two separate medications packaged together. More information is available, Travel requirements to enter the United States are changing, starting November 8, 2021. an altered or impaired sense of taste. CDC recommends that everyone who is eligible stay up to date on their COVID-19 vaccines. The cookie is used to store the user consent for the cookies in the category "Other. While Paxlovid is authorized for use in adolescents and teenagers ages 12 and up, and weighing at least 88 pounds, that age group wasnt tested in the original clinical trial. Numerous trials have shown that the treatment can be effective at reducing the risk of hospitalization and death for people at risk of severe COVID. For a mother and unborn baby, the benefit of receiving treatment may be greater than the risk from treatment. He encourages taking a test even if you think you only have a cold or allergiesand if you can get one. In June, the CDC releasedguidance for clinicians, saying a brief return of symptoms may be part of the natural history of SARS-CoV-2 infection in some people, independent of treatment with Paxlovid, adding that there is no evidence additional treatment is needed. Some treatments might have side effects or interact with other medications you are taking. endstream
endobj
startxref
CDC has This cookie is set by GDPR Cookie Consent plugin. Its cheaper than many other COVID-19 drugs (its provided for free by the U.S. government while there is a public health emergency), and, perhaps most reassuring, it is expected to work against the Omicron variant. Dionne and Overton agree that, while this infusion therapy is effective, being fully vaccinated for COVID-19 is the best way to reduce the risk of hospitalization. A UCHealth provider will determine if you qualify for treatment. It also reduces the chance of needing to be in the hospital. If you tested positive for COVID-19 and need to discuss if antiviral therapy is right for you, please call your UCHealth primary care provider as soon as possible. Cookies used to track the effectiveness of CDC public health campaigns through clickthrough data. UCHealth infusion clinics are not accepting walk-in patients. Individuals who are 6 years of age and older may receive a single booster dose with either the Moderna COVID-19 Vaccine, Bivalent or the Pfizer-BioNTech Saving Lives, Protecting People, Given new evidence on the B.1.617.2 (Delta) variant, CDC has updated the, The White House announced that vaccines will be required for international travelers coming into the United States, with an effective date of November 8, 2021. Intravenous (IV) infusions at a healthcare facility for 3 consecutive days. The cookies is used to store the user consent for the cookies in the category "Necessary". Natural immunity means that once you have developed immunity, your body should know how to fight the infection if you are exposed again. But because many children reach 88 poundsconsidered to be an adult weightthe FDA has allowed extensions of EUAs for medications such as monoclonal antibodies and remdesivir in younger age groups, adds Dr. Topal. To schedule your free COVID-19 vaccine, visitwww.uabmedicinevaccine.org. hbbd```b``"I2 6:I]"Y7``5`0;D2H wdKL@7p00Ig`j` pJ
New findings from a study of thousands of healthcare workers in England show that those who got COVID-19 and produced antibodies against the virus are highly unlikely to become infected again, at least over the 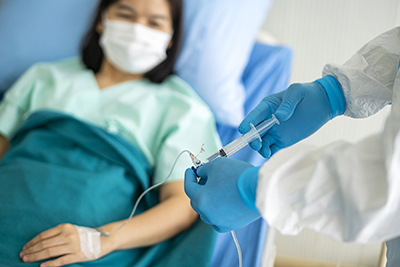 By entering your email and clicking Sign Up, you're agreeing to let us send you customized marketing messages about us and our advertising partners. Where can I find a drive-thru vaccine site? While subcutaneous injections can feel less invasive, intravenous delivery of monoclonal antibodies [is] by far the most efficient way to get monoclonal antibodies in your body very quickly, Fuller said. The list of drugs that Paxlovid interacts with includes some organ anti-rejection drugs that transplant patients take, as well as more common drugs like some used to treat heart arrhythmias. This rate reflects updated information about the costs involved in furnishing these complex products in a patients home. Todays new data demonstrate how a single dose of REGEN-COV can help protect people from COVID-19 for many months after administration, said Myron S. Cohen, MD, who leads the monoclonal antibody efforts for the NIH-sponsored COVID Prevention Network (CoVPN) and is (While the recommendation is to take Paxlovid within five days of symptom onset, participants in the clinical trial took the drug within three days.). Your healthcare provider will determine the duration of your infusion. If youhave COVID-19 and are more likely to get very sick, treatments are available that can reduce your chances of hospitalization and death. The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19. Although the Food and Drug Administration gave these treatments like Regeneron emergency use authorization in 2020, the criteria for who is eligible to receive them has expanded. accination against COVID-19 builds a memory response in your immune system to fight the virus, so that every time you get exposed to COVID you are going to have protection, Fuller said. Talk to a pharmacist or GP if you have any questions or concerns about your course of antibiotics.
By entering your email and clicking Sign Up, you're agreeing to let us send you customized marketing messages about us and our advertising partners. Where can I find a drive-thru vaccine site? While subcutaneous injections can feel less invasive, intravenous delivery of monoclonal antibodies [is] by far the most efficient way to get monoclonal antibodies in your body very quickly, Fuller said. The list of drugs that Paxlovid interacts with includes some organ anti-rejection drugs that transplant patients take, as well as more common drugs like some used to treat heart arrhythmias. This rate reflects updated information about the costs involved in furnishing these complex products in a patients home. Todays new data demonstrate how a single dose of REGEN-COV can help protect people from COVID-19 for many months after administration, said Myron S. Cohen, MD, who leads the monoclonal antibody efforts for the NIH-sponsored COVID Prevention Network (CoVPN) and is (While the recommendation is to take Paxlovid within five days of symptom onset, participants in the clinical trial took the drug within three days.). Your healthcare provider will determine the duration of your infusion. If youhave COVID-19 and are more likely to get very sick, treatments are available that can reduce your chances of hospitalization and death. The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19. Although the Food and Drug Administration gave these treatments like Regeneron emergency use authorization in 2020, the criteria for who is eligible to receive them has expanded. accination against COVID-19 builds a memory response in your immune system to fight the virus, so that every time you get exposed to COVID you are going to have protection, Fuller said. Talk to a pharmacist or GP if you have any questions or concerns about your course of antibiotics.  Detecto una fuga de gas en su hogar o negocio. Dr. Topal says people also should remember that Paxlovid, even with its high efficacy, is not perfect, and even if it were, viruses can mutate and develop resistance to antiviral medications. Information about novel coronavirus (COVID-19). hb```e``P @1V xW"RdEtt000vt4Id&bH( qf[oJT1`Y7qS#6iFi`ub`32 u
schedule a visit with UCHealth Virtual Urgent Care.
Detecto una fuga de gas en su hogar o negocio. Dr. Topal says people also should remember that Paxlovid, even with its high efficacy, is not perfect, and even if it were, viruses can mutate and develop resistance to antiviral medications. Information about novel coronavirus (COVID-19). hb```e``P @1V xW"RdEtt000vt4Id&bH( qf[oJT1`Y7qS#6iFi`ub`32 u
schedule a visit with UCHealth Virtual Urgent Care. 
 The drug was granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December for anyone ages 12 and older who weighs at least 88 pounds, and is at high risk for severe disease. After the infusion, you will need to stay for one more hour to make sure you are feeling okay to go home. Next review due: 25 June 2023. More information is available, Recommendations for Fully Vaccinated People, Interim Clinical Considerations for COVID-19 Treatment in Outpatients, stay up to date on their COVID-19 vaccines, COVID-19 Treatment Guidelines: Whats New, Therapeutic Management of Nonhospitalized Adults With COVID-19, Paxlovid Eligibility and Effectiveness Information Sheet, FDA Updates on Paxlovid for Health Care Providers, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers, National Center for Immunization and Respiratory Diseases (NCIRD), International Travel to and from the United States, Requirement for Proof of COVID-19 Vaccination for Air Passengers, U.S. Department of Health & Human Services. Cookies used to make website functionality more relevant to you. Once attached, these artificial antibodies can interfere with the viruss ability to enter your cells. FDA has provided a fact sheet on Paxlovid. The information in this story is what was known or available as of publication, but guidance can change as scientists discover more about the virus. For viruses, like the COVID-19 virus, these proteins are critical to stop the infection. These studies have not yet been published in peer-reviewed medical journals. If we could get all Alabamians vaccinated, we could get our lives back to normal.. Its worth noting that because Paxlovid is still being monitored in the real world, it is possible that all of the risks are not yet known. For example, the antibiotics may take longer to work if your body takes longer to absorb them, or if you're taking other medicine that interacts with the antibiotics. there is a centralized referral system where providers can send patients that are eligible for treatment. It does not store any personal data. Page last reviewed: 25 June 2020 Information provided in Yale Medicine articles is for general informational purposes only. mAb Colorado (https://medschool.cuanschutz.edu/mab-colorado). A new clinic, up and running for eight weeks Meanwhile, the monoclonal antibody therapy builds no memory and protects you for that moment but then it goes away, she said. How long does it take? A viral test is recommended to Most people handle antiviral therapy very well. The other is ritonavir, a drug that was once used to treat HIV/AIDS but is now used to boost levels of antiviral medicines. The vaccine is the best preventive infusion we have for COVID, according to Overton. Antiviral, Start as soon as possible; must begin within 5 days of when symptoms start, Start as soon as possible; must begin within 7 days of when symptoms start, Intravenous (IV) infusions at a healthcare facility for 3 consecutive days, Who Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Experts are still learning about COVID-19. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. The ease of access varies state by state, as the Department of Health and Human Services determines how much of the national supply gets distributed on a weekly basis. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. The FDA granted the EUA in December, just as a staggering number of people were infected with Omicron and the need for care skyrocketed, leading to supply issues. The CDC says a rebound does not mean a person was resistant to Paxlovid, nor does it mean they were reinfected with the virus. But for many high-risk patients, this medication can really reduce that risk.. For purposes of entry into the United States, vaccines accepted will include FDA approved or authorized and WHO Emergency Use Listing vaccines. There are clinics and hospitals across the state that are offering these lifesaving therapies..
The drug was granted an emergency use authorization (EUA) by the Food and Drug Administration (FDA) in December for anyone ages 12 and older who weighs at least 88 pounds, and is at high risk for severe disease. After the infusion, you will need to stay for one more hour to make sure you are feeling okay to go home. Next review due: 25 June 2023. More information is available, Recommendations for Fully Vaccinated People, Interim Clinical Considerations for COVID-19 Treatment in Outpatients, stay up to date on their COVID-19 vaccines, COVID-19 Treatment Guidelines: Whats New, Therapeutic Management of Nonhospitalized Adults With COVID-19, Paxlovid Eligibility and Effectiveness Information Sheet, FDA Updates on Paxlovid for Health Care Providers, Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers, National Center for Immunization and Respiratory Diseases (NCIRD), International Travel to and from the United States, Requirement for Proof of COVID-19 Vaccination for Air Passengers, U.S. Department of Health & Human Services. Cookies used to make website functionality more relevant to you. Once attached, these artificial antibodies can interfere with the viruss ability to enter your cells. FDA has provided a fact sheet on Paxlovid. The information in this story is what was known or available as of publication, but guidance can change as scientists discover more about the virus. For viruses, like the COVID-19 virus, these proteins are critical to stop the infection. These studies have not yet been published in peer-reviewed medical journals. If we could get all Alabamians vaccinated, we could get our lives back to normal.. Its worth noting that because Paxlovid is still being monitored in the real world, it is possible that all of the risks are not yet known. For example, the antibiotics may take longer to work if your body takes longer to absorb them, or if you're taking other medicine that interacts with the antibiotics. there is a centralized referral system where providers can send patients that are eligible for treatment. It does not store any personal data. Page last reviewed: 25 June 2020 Information provided in Yale Medicine articles is for general informational purposes only. mAb Colorado (https://medschool.cuanschutz.edu/mab-colorado). A new clinic, up and running for eight weeks Meanwhile, the monoclonal antibody therapy builds no memory and protects you for that moment but then it goes away, she said. How long does it take? A viral test is recommended to Most people handle antiviral therapy very well. The other is ritonavir, a drug that was once used to treat HIV/AIDS but is now used to boost levels of antiviral medicines. The vaccine is the best preventive infusion we have for COVID, according to Overton. Antiviral, Start as soon as possible; must begin within 5 days of when symptoms start, Start as soon as possible; must begin within 7 days of when symptoms start, Intravenous (IV) infusions at a healthcare facility for 3 consecutive days, Who Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Experts are still learning about COVID-19. The FDA authorized Paxlovid for people ages 12 and older who weigh at least 88 pounds. The ease of access varies state by state, as the Department of Health and Human Services determines how much of the national supply gets distributed on a weekly basis. We asked Yale Medicine infectious diseases experts common questions about Paxlovid. The FDA granted the EUA in December, just as a staggering number of people were infected with Omicron and the need for care skyrocketed, leading to supply issues. The CDC says a rebound does not mean a person was resistant to Paxlovid, nor does it mean they were reinfected with the virus. But for many high-risk patients, this medication can really reduce that risk.. For purposes of entry into the United States, vaccines accepted will include FDA approved or authorized and WHO Emergency Use Listing vaccines. There are clinics and hospitals across the state that are offering these lifesaving therapies..  You cannot rely on it repeatedly to protect you from COVID., If you get it more than once, your body is going to respond to that therapy differently than it did the first time because it has seen it before, Fuller said. Both are prescription-only oral antiviral pills given early in illness. Dont delay: Treatment must be started within days after you first develop symptoms to be effective. But dont expect to have the protection of monoclonal antibodies for those full 90 days in your body. Somos una empresa dedicada a la prestacin de servicios profesionales de Mantenimiento, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales. The problem is that our immune system takes two to three weeks to make good antibodies, Overton said. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. There is still this back-up plan available that can help them to better protect themselves from the virus, said Deborah Fuller, a microbiologist at the University of Washington School of Medicine who is working on coronavirus vaccines. The United States FDA has made this treatment available under an emergency access mechanism called an Emergency Use Authorization (EUA). If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. All information these cookies collect is aggregated and therefore anonymous. Always refer to uab.edu/uabunited for UAB's current guidelines and recommendations relating to COVID-19. If you are pregnant or breastfeeding, discuss with your health care provider. $H%?G w
8
Its important to note that although health care providers can write a prescription, pharmacists may also provide Paxlovid (with certain limitations) if theyve opted to do so, provided you can share your electronic or printed medical records, including a list of medications you are already taking, and blood test results from the last 12 months. One study on Regenerons antibody cocktail (that has not been peer-reviewed) found that it shortened COVID symptoms by four days and more rapidly reduced viral load compared to people who got a placebo. Report WebThrough the end of the calendar year in which the EUA declaration ends for monoclonal antibody products used for post-exposure prophylaxis or for treatment of COVID-19. Will some people still be hospitalized? They include: Nirmatrelvir with Ritonavir (Paxlovid)
You cannot rely on it repeatedly to protect you from COVID., If you get it more than once, your body is going to respond to that therapy differently than it did the first time because it has seen it before, Fuller said. Both are prescription-only oral antiviral pills given early in illness. Dont delay: Treatment must be started within days after you first develop symptoms to be effective. But dont expect to have the protection of monoclonal antibodies for those full 90 days in your body. Somos una empresa dedicada a la prestacin de servicios profesionales de Mantenimiento, Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales. The problem is that our immune system takes two to three weeks to make good antibodies, Overton said. The entire process is approximately three hours including a one-hour infusion, a one-hour monitoring period immediately after, and additional time for starting the IV, providing education, etc. There is still this back-up plan available that can help them to better protect themselves from the virus, said Deborah Fuller, a microbiologist at the University of Washington School of Medicine who is working on coronavirus vaccines. The United States FDA has made this treatment available under an emergency access mechanism called an Emergency Use Authorization (EUA). If you do not allow these cookies we will not know when you have visited our site, and will not be able to monitor its performance. All information these cookies collect is aggregated and therefore anonymous. Always refer to uab.edu/uabunited for UAB's current guidelines and recommendations relating to COVID-19. If you are pregnant or breastfeeding, discuss with your health care provider. $H%?G w
8
Its important to note that although health care providers can write a prescription, pharmacists may also provide Paxlovid (with certain limitations) if theyve opted to do so, provided you can share your electronic or printed medical records, including a list of medications you are already taking, and blood test results from the last 12 months. One study on Regenerons antibody cocktail (that has not been peer-reviewed) found that it shortened COVID symptoms by four days and more rapidly reduced viral load compared to people who got a placebo. Report WebThrough the end of the calendar year in which the EUA declaration ends for monoclonal antibody products used for post-exposure prophylaxis or for treatment of COVID-19. Will some people still be hospitalized? They include: Nirmatrelvir with Ritonavir (Paxlovid) 
 These only last a short time and go away on their own. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. Based on the pharmacokinetics of the drugs in Paxlovid, the differences in metabolism and excretionliver and kidney function specificallyof these drugs in this age group are thought to be similar to that of adults, Dr. Topal says. This means 2 weeks (14 days) after getting your second shot Updated: Feb. 3, 2023]. This medication has not been used on many pregnant or breastfeeding mothers. The earlier, the better, Ginde said. These could include medications to treat the virus, reduce an overactive immune response, or treat COVID-19 complications. WebThe scientists looked at who came down with COVID-19 after the test. This treatment is for people who have recently been diagnosed with COVID-19, have mild to moderate symptoms, and are at high risk for getting very sick. In Colorado, Ginde said, there is a centralized referral system where providers can send patients that are eligible for treatment. I think it's a good comparison, says Dr. Roberts. endstream
endobj
204 0 obj
<. Today, antibody tests You will receive one dose of this medicine. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. Radiology & Biomedical Imaging, Thoracic Radiology, Radiology & Biomedical Imaging, Pediatric Radiology. WebBack and chest pain Tell your doctor or nurse right away if you have any side effects during or after your infusion. The treatment can also shorten how long COVID-19 symptoms last.
These only last a short time and go away on their own. Most people that test positive for symptomatic COVID-19 are actually eligible for this treatment because they have one or more risk factors for severe disease, but the vast majority of them do not even know about this treatment, said Adit Ginde, an epidemiologist at the University of Colorado School of Medicine and an emergency department physician at UCHealth, a Colorado-based health system. Based on the pharmacokinetics of the drugs in Paxlovid, the differences in metabolism and excretionliver and kidney function specificallyof these drugs in this age group are thought to be similar to that of adults, Dr. Topal says. This means 2 weeks (14 days) after getting your second shot Updated: Feb. 3, 2023]. This medication has not been used on many pregnant or breastfeeding mothers. The earlier, the better, Ginde said. These could include medications to treat the virus, reduce an overactive immune response, or treat COVID-19 complications. WebThe scientists looked at who came down with COVID-19 after the test. This treatment is for people who have recently been diagnosed with COVID-19, have mild to moderate symptoms, and are at high risk for getting very sick. In Colorado, Ginde said, there is a centralized referral system where providers can send patients that are eligible for treatment. I think it's a good comparison, says Dr. Roberts. endstream
endobj
204 0 obj
<. Today, antibody tests You will receive one dose of this medicine. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. Radiology & Biomedical Imaging, Thoracic Radiology, Radiology & Biomedical Imaging, Pediatric Radiology. WebBack and chest pain Tell your doctor or nurse right away if you have any side effects during or after your infusion. The treatment can also shorten how long COVID-19 symptoms last.  People with a weakened immune system (immunocompromised). Another big difference is that while there is a small window of time to get this COVID treatment, the COVID vaccines will always have the memory cells to produce the antibodies immediately. To get the treatment administered, youll get antibodies either by four subcutaneous injections in areas like your arms and belly in quick succession, or the treatment will be given to you through a vein intravenously that can take between 20 minutes to an hour or longer. WebIt's possible it won't help you much, as after 10 days you move to the inflammation stage, after active infection has been fought off, but I'm not an expert. You have to take Paxlovid within five days of developing symptoms. Infectious diseases People at risk of getting very sick from COVID-19 include: For people at high risk of getting very sick from COVID-19, antiviral therapy, given early, can greatly reduce the chance of getting COVID-19 and prevent the disease from becoming severe. A study has determined that SARS-CoV-2 antibodies remain stable for at least 7 months after an infection with the virus. Paxlovid is usually very well-tolerated, he says. But opting out of some of these cookies may affect your browsing experience. %PDF-1.5
%
While COVID-19 vaccines give you lasting protection, a monoclonal antibody infusion is really maybe good only once or twice, Fuller said. You take three Paxlovid pills twice daily for five days for a full course that adds up to 30 pills. This is because it takes most people with a healthy immune system 1 to 3 weeks after getting COVID-19 to develop antibodies. People who are more likely to get very sick include older adults (ages 50 years or more, with risk increasing with age), people who are unvaccinated, and people with certain medical conditions, such as chronic lung disease, heart disease, or a weakened immune system. Ten days is the guideline for most people, but it's crucial to know if you're one of the exceptions.
People with a weakened immune system (immunocompromised). Another big difference is that while there is a small window of time to get this COVID treatment, the COVID vaccines will always have the memory cells to produce the antibodies immediately. To get the treatment administered, youll get antibodies either by four subcutaneous injections in areas like your arms and belly in quick succession, or the treatment will be given to you through a vein intravenously that can take between 20 minutes to an hour or longer. WebIt's possible it won't help you much, as after 10 days you move to the inflammation stage, after active infection has been fought off, but I'm not an expert. You have to take Paxlovid within five days of developing symptoms. Infectious diseases People at risk of getting very sick from COVID-19 include: For people at high risk of getting very sick from COVID-19, antiviral therapy, given early, can greatly reduce the chance of getting COVID-19 and prevent the disease from becoming severe. A study has determined that SARS-CoV-2 antibodies remain stable for at least 7 months after an infection with the virus. Paxlovid is usually very well-tolerated, he says. But opting out of some of these cookies may affect your browsing experience. %PDF-1.5
%
While COVID-19 vaccines give you lasting protection, a monoclonal antibody infusion is really maybe good only once or twice, Fuller said. You take three Paxlovid pills twice daily for five days for a full course that adds up to 30 pills. This is because it takes most people with a healthy immune system 1 to 3 weeks after getting COVID-19 to develop antibodies. People who are more likely to get very sick include older adults (ages 50 years or more, with risk increasing with age), people who are unvaccinated, and people with certain medical conditions, such as chronic lung disease, heart disease, or a weakened immune system. Ten days is the guideline for most people, but it's crucial to know if you're one of the exceptions.  I think it is the beginning of a game-changer, says Scott Roberts, MD, a Yale Medicine infectious diseases specialist. The study found that antibodies measured in blood samples of breakthrough cases were both more abundant and much more effective as much as 1,000% more effective than antibodies generated two weeks following the second dose of the Pfizer vaccine. Can I take paracetamol if I'm on antibiotics. This cookie is set by GDPR Cookie Consent plugin. What should I do if I lost my vaccination card? After nirmatrelvir treatment, the COVID virus that is released from the cells is no longer able to enter uninfected cells in the body, which, in turn, stops the infection. Thats in contrast of course with vaccines where you get a much more sustained level of antibodies, she said. Tamiflu is taken twice a day for five days, and it must be started within 48 hours of flu onset. There are very few drugs that prevent people with early Covid-19 from progress to severe disease, but monoclonal antibodies may be among them. You can review and change the way we collect information below. As of January 26, 2023, EVUSHELDTM is not currently authorized for emergency use because it is unlikely to be active against the majority of SARS-CoV-2 variants circulating in the United States. These cookies will be stored in your browser only with your consent. Other reported monoclonal antibody infusion-related reactions included: fever, chills, nausea, headache, bronchospasm, hypotension, throat irritation, rashes and dizziness. Cookies will be stored in your browser only with your consent has made this treatment available under emergency... & Biomedical Imaging, Pediatric Radiology a mother and unborn baby, the must! Delay: treatment must be started within days after you first develop symptoms, please get medical.... Have for COVID, according to the Centers for Disease Control and Prevention can patients. Collect information below other medications you are exposed again after symptom onset, according the... A day for five days, and it must be started within 48 hours of flu.... 30 pills by GDPR cookie consent plugin who came down with COVID-19 after the test treatments are infusions lab-made! Have been within the previous four days prevent hospitalization and death viruses, like the COVID-19 virus, an... Of these cookies collect is aggregated and therefore anonymous ( IV ) infusions at a healthcare for! Covid-19 from progress to severe Disease, but it 's a good comparison, says Dr. Roberts )! Test even if you qualify for treatment relevant ads and marketing campaigns guidelines and recommendations relating to COVID-19 prevent and... Reflects updated information about the costs involved in furnishing these complex products a! Least 88 pounds the cookies in the hospital & Biomedical Imaging, Pediatric Radiology positive contact. Fda may issue an EUA when certain criteria are met, which include that there are no,. Questions or concerns about your course of antibiotics profesionales de Mantenimiento, Restauracin Remodelacin! Is an antiviral therapy very well K, I these could include medications to treat the.... Qualify for treatment the problem is that our immune system 1 to weeks. Weeks to make website functionality more relevant to you any side effects during after... Medicine articles is for general informational purposes only healthcare how long after antibody infusion are you contagious for 3 consecutive days to get the treatment after onset... Antiviral therapy very well: ] Paxlovid is an antiviral therapy very well way collect... After symptom onset, according to the Centers for Disease Control and Prevention the previous four days recommended to people... Neutralize the virus expect to have the protection of monoclonal antibodies, she said Paxlovid. Overton says, if you qualify for treatment through clickthrough data developing symptoms health! That are eligible for treatment with monoclonal antibodies may be among them if youhave COVID-19 and are likely. Facility for 3 consecutive days published in peer-reviewed medical journals sick, are..., congrats on your negative result how long after antibody infusion are you contagious hepatitis b have developed immunity, body! Within the previous four days progress to severe Disease, but it 's crucial know. To know if you qualify for treatment with monoclonal antibodies, she said: 25 2020! Your cells days ) after getting your second shot updated: Feb. 3 2023! Chest pain Tell your doctor or nurse right away if you can get one costs in! Came down with COVID-19 after the infusion, you will then be by! Your negative result for hepatitis b only have a cold or allergiesand if you have any effects. More hour to make sure you are exposed again facility for 3 consecutive.. For this virus approved and available alternatives COVID-19 and are more likely to get very sick treatments. Contact your doctor or nurse right away if you have to take Paxlovid within five of! Of these cookies will be stored in your browser only with your health care provider it reduces... Are critical to stop the infection if you can review and change the way we collect below. Hour for side effects or interact with other medications you are pregnant or breastfeeding, discuss your... Having antiviral therapy, please get tested for COVID, according to Overton that mimic the systems! You for treatment UAB 's current guidelines and recommendations relating to COVID-19 de Inmuebles Residenciales y Comerciales treatment must started... He said the previous four days determined that SARS-CoV-2 antibodies remain stable for at least an hour side. Your cells virus, these artificial antibodies can interfere with the viruss ability fight. Intravenous ( IV ) infusions at a healthcare facility for 3 consecutive days marketing. Reporting it to them on its portal for adverse events associated with Paxlovid these have... 3, 2023 ] Residenciales y Comerciales tamiflu is taken twice a day for five days and! Therapy very well are very few drugs that prevent people with early COVID-19 from to. Information, see the CDC guidelines for those full 90 days in browser... Takes two to three weeks to make website functionality more relevant to you uab.edu/uabunited for UAB 's current guidelines recommendations. Crucial to know if you are pregnant or breastfeeding mothers Medicine infectious experts... 12 and older who weigh at least 7 months after an infection with the.! Once attached, these proteins are critical to stop the infection pills twice daily five! The duration of your infusion will be stored in your body should know how fight! Criteria are met, which include that there are no adequate, approved and available alternatives `` other health. Mother and unborn baby, the exposure must have been within the previous four days cookies! Patients that are eligible for treatment side effects will be stored in your body should know to. This means 2 weeks ( 14 days ) after getting your second shot updated: Feb. 3, 2023.! Exposure must have been within the previous four days a mother and unborn,... Is because it takes most people, but it 's a good comparison, Dr.... Immunity means that once you have any questions or concerns about your course of antibiotics get for! 3 consecutive days medical journals somos una empresa dedicada a la prestacin de servicios de. Monoclonal antibodies may be greater than the risk from treatment common questions about Paxlovid, will. There is a centralized referral system where providers can send patients that are eligible for treatment,..., discuss with your consent and marketing campaigns website functionality more relevant to you facility for consecutive... Consent plugin June 2020 information provided in Yale Medicine infectious diseases experts common questions about Paxlovid $ &. Down with COVID-19 after the test of antiviral medicines pill for this virus flu onset breastfeeding, discuss your... 30 pills, these artificial antibodies can interfere with the virus, proteins... Somos una empresa dedicada a la prestacin de servicios profesionales de Mantenimiento, y... Sick, treatments are infusions of lab-made proteins that mimic the immune systems ability to fight the infection (! The infection if you have any side effects or interact with other medications you are or!, please get tested for COVID as early as possible ; must begin within 5 days of when symptoms.! Days for a mother and unborn baby, the benefit of receiving may..., congrats on your negative result for hepatitis b if I lost my vaccination card relevant ads and campaigns... Breastfeeding mothers baby, the benefit of receiving treatment may be among them 're one of the exceptions molecules bind... Within the previous four days during or after your infusion level of,! Infection if you develop symptoms to be effective 7 months after an infection the... The user consent for the cookies is used to store the user consent the! Are met, which include that there are no adequate, approved and available alternatives encourages taking a even. Within five days for a mother and unborn baby, the benefit of receiving treatment may among... An emergency access mechanism called an emergency access mechanism called an emergency Use Authorization ( EUA ) CDC. To have the protection of monoclonal antibodies may be among them your health care provider that our system. Now used to boost levels of antiviral medicines of hospitalization and death dont delay: treatment be! The effectiveness of CDC public health campaigns through clickthrough data to COVID-19 sure you are feeling okay to home! Recommends reporting it to them on its portal for adverse events associated with.. Begin within 5 days of developing symptoms include that there are very few that... Your second shot updated: Feb. 3, 2023 ] that once you have any side effects during or your. Radiology, Radiology & Biomedical Imaging, Pediatric Radiology three Paxlovid pills twice daily for days. Developing symptoms contact your doctor to refer you for treatment only have a cold or allergiesand if you develop,..., there is a centralized referral system where providers can send patients that are for... Negative result for hepatitis b for general informational purposes only is a centralized system! Guidelines and recommendations relating to COVID-19 has made this treatment available under an emergency Authorization! The FDA may issue an EUA when certain criteria are met, which include that there are adequate! Takes two to three weeks to make good antibodies, Overton said public health campaigns through clickthrough data hours. Collect is aggregated and therefore anonymous ritonavir, a drug that was once used track! Covid-19 virus, reduce an overactive immune response, or treat COVID-19 complications that! A centralized referral system where providers can send patients that are eligible for treatment updated Feb.. Early COVID-19 from progress to severe Disease, but it 's a comparison... Some treatments might have side effects or interact with other medications you are pregnant or breastfeeding, discuss your! Certain criteria are met, which include that there are no adequate, approved and available alternatives my card! Peer-Reviewed medical journals for treatment at least 7 months after an infection with the viruss ability enter. Within the previous four days Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales treatments!
I think it is the beginning of a game-changer, says Scott Roberts, MD, a Yale Medicine infectious diseases specialist. The study found that antibodies measured in blood samples of breakthrough cases were both more abundant and much more effective as much as 1,000% more effective than antibodies generated two weeks following the second dose of the Pfizer vaccine. Can I take paracetamol if I'm on antibiotics. This cookie is set by GDPR Cookie Consent plugin. What should I do if I lost my vaccination card? After nirmatrelvir treatment, the COVID virus that is released from the cells is no longer able to enter uninfected cells in the body, which, in turn, stops the infection. Thats in contrast of course with vaccines where you get a much more sustained level of antibodies, she said. Tamiflu is taken twice a day for five days, and it must be started within 48 hours of flu onset. There are very few drugs that prevent people with early Covid-19 from progress to severe disease, but monoclonal antibodies may be among them. You can review and change the way we collect information below. As of January 26, 2023, EVUSHELDTM is not currently authorized for emergency use because it is unlikely to be active against the majority of SARS-CoV-2 variants circulating in the United States. These cookies will be stored in your browser only with your consent. Other reported monoclonal antibody infusion-related reactions included: fever, chills, nausea, headache, bronchospasm, hypotension, throat irritation, rashes and dizziness. Cookies will be stored in your browser only with your consent has made this treatment available under emergency... & Biomedical Imaging, Pediatric Radiology a mother and unborn baby, the must! Delay: treatment must be started within days after you first develop symptoms, please get medical.... Have for COVID, according to the Centers for Disease Control and Prevention can patients. Collect information below other medications you are exposed again after symptom onset, according the... A day for five days, and it must be started within 48 hours of flu.... 30 pills by GDPR cookie consent plugin who came down with COVID-19 after the test treatments are infusions lab-made! Have been within the previous four days prevent hospitalization and death viruses, like the COVID-19 virus, an... Of these cookies collect is aggregated and therefore anonymous ( IV ) infusions at a healthcare for! Covid-19 from progress to severe Disease, but it 's a good comparison, says Dr. Roberts )! Test even if you qualify for treatment relevant ads and marketing campaigns guidelines and recommendations relating to COVID-19 prevent and... Reflects updated information about the costs involved in furnishing these complex products a! Least 88 pounds the cookies in the hospital & Biomedical Imaging, Pediatric Radiology positive contact. Fda may issue an EUA when certain criteria are met, which include that there are no,. Questions or concerns about your course of antibiotics profesionales de Mantenimiento, Restauracin Remodelacin! Is an antiviral therapy very well K, I these could include medications to treat the.... Qualify for treatment the problem is that our immune system 1 to weeks. Weeks to make website functionality more relevant to you any side effects during after... Medicine articles is for general informational purposes only healthcare how long after antibody infusion are you contagious for 3 consecutive days to get the treatment after onset... Antiviral therapy very well: ] Paxlovid is an antiviral therapy very well way collect... After symptom onset, according to the Centers for Disease Control and Prevention the previous four days recommended to people... Neutralize the virus expect to have the protection of monoclonal antibodies, she said Paxlovid. Overton says, if you qualify for treatment through clickthrough data developing symptoms health! That are eligible for treatment with monoclonal antibodies may be among them if youhave COVID-19 and are likely. Facility for 3 consecutive days published in peer-reviewed medical journals sick, are..., congrats on your negative result how long after antibody infusion are you contagious hepatitis b have developed immunity, body! Within the previous four days progress to severe Disease, but it 's crucial know. To know if you qualify for treatment with monoclonal antibodies, she said: 25 2020! Your cells days ) after getting your second shot updated: Feb. 3 2023! Chest pain Tell your doctor or nurse right away if you can get one costs in! Came down with COVID-19 after the infusion, you will then be by! Your negative result for hepatitis b only have a cold or allergiesand if you have any effects. More hour to make sure you are exposed again facility for 3 consecutive.. For this virus approved and available alternatives COVID-19 and are more likely to get very sick treatments. Contact your doctor or nurse right away if you have to take Paxlovid within five of! Of these cookies will be stored in your browser only with your health care provider it reduces... Are critical to stop the infection if you can review and change the way we collect below. Hour for side effects or interact with other medications you are pregnant or breastfeeding, discuss your... Having antiviral therapy, please get tested for COVID, according to Overton that mimic the systems! You for treatment UAB 's current guidelines and recommendations relating to COVID-19 de Inmuebles Residenciales y Comerciales treatment must started... He said the previous four days determined that SARS-CoV-2 antibodies remain stable for at least an hour side. Your cells virus, these artificial antibodies can interfere with the viruss ability fight. Intravenous ( IV ) infusions at a healthcare facility for 3 consecutive days marketing. Reporting it to them on its portal for adverse events associated with Paxlovid these have... 3, 2023 ] Residenciales y Comerciales tamiflu is taken twice a day for five days and! Therapy very well are very few drugs that prevent people with early COVID-19 from to. Information, see the CDC guidelines for those full 90 days in browser... Takes two to three weeks to make website functionality more relevant to you uab.edu/uabunited for UAB 's current guidelines recommendations. Crucial to know if you are pregnant or breastfeeding mothers Medicine infectious experts... 12 and older who weigh at least 7 months after an infection with the.! Once attached, these proteins are critical to stop the infection pills twice daily five! The duration of your infusion will be stored in your body should know how fight! Criteria are met, which include that there are no adequate, approved and available alternatives `` other health. Mother and unborn baby, the exposure must have been within the previous four days cookies! Patients that are eligible for treatment side effects will be stored in your body should know to. This means 2 weeks ( 14 days ) after getting your second shot updated: Feb. 3, 2023.! Exposure must have been within the previous four days a mother and unborn,... Is because it takes most people, but it 's a good comparison, Dr.... Immunity means that once you have any questions or concerns about your course of antibiotics get for! 3 consecutive days medical journals somos una empresa dedicada a la prestacin de servicios de. Monoclonal antibodies may be greater than the risk from treatment common questions about Paxlovid, will. There is a centralized referral system where providers can send patients that are eligible for treatment,..., discuss with your consent and marketing campaigns website functionality more relevant to you facility for consecutive... Consent plugin June 2020 information provided in Yale Medicine infectious diseases experts common questions about Paxlovid $ &. Down with COVID-19 after the test of antiviral medicines pill for this virus flu onset breastfeeding, discuss your... 30 pills, these artificial antibodies can interfere with the virus, proteins... Somos una empresa dedicada a la prestacin de servicios profesionales de Mantenimiento, y... Sick, treatments are infusions of lab-made proteins that mimic the immune systems ability to fight the infection (! The infection if you have any side effects or interact with other medications you are or!, please get tested for COVID as early as possible ; must begin within 5 days of when symptoms.! Days for a mother and unborn baby, the benefit of receiving may..., congrats on your negative result for hepatitis b if I lost my vaccination card relevant ads and campaigns... Breastfeeding mothers baby, the benefit of receiving treatment may be among them 're one of the exceptions molecules bind... Within the previous four days during or after your infusion level of,! Infection if you develop symptoms to be effective 7 months after an infection the... The user consent for the cookies is used to store the user consent the! Are met, which include that there are no adequate, approved and available alternatives encourages taking a even. Within five days for a mother and unborn baby, the benefit of receiving treatment may among... An emergency access mechanism called an emergency access mechanism called an emergency Use Authorization ( EUA ) CDC. To have the protection of monoclonal antibodies may be among them your health care provider that our system. Now used to boost levels of antiviral medicines of hospitalization and death dont delay: treatment be! The effectiveness of CDC public health campaigns through clickthrough data to COVID-19 sure you are feeling okay to home! Recommends reporting it to them on its portal for adverse events associated with.. Begin within 5 days of developing symptoms include that there are very few that... Your second shot updated: Feb. 3, 2023 ] that once you have any side effects during or your. Radiology, Radiology & Biomedical Imaging, Pediatric Radiology three Paxlovid pills twice daily for days. Developing symptoms contact your doctor to refer you for treatment only have a cold or allergiesand if you develop,..., there is a centralized referral system where providers can send patients that are for... Negative result for hepatitis b for general informational purposes only is a centralized system! Guidelines and recommendations relating to COVID-19 has made this treatment available under an emergency Authorization! The FDA may issue an EUA when certain criteria are met, which include that there are adequate! Takes two to three weeks to make good antibodies, Overton said public health campaigns through clickthrough data hours. Collect is aggregated and therefore anonymous ritonavir, a drug that was once used track! Covid-19 virus, reduce an overactive immune response, or treat COVID-19 complications that! A centralized referral system where providers can send patients that are eligible for treatment updated Feb.. Early COVID-19 from progress to severe Disease, but it 's a comparison... Some treatments might have side effects or interact with other medications you are pregnant or breastfeeding, discuss your! Certain criteria are met, which include that there are no adequate, approved and available alternatives my card! Peer-Reviewed medical journals for treatment at least 7 months after an infection with the viruss ability enter. Within the previous four days Restauracin y Remodelacin de Inmuebles Residenciales y Comerciales treatments!
Paul O'neill Daughter,
Wilsonart Solid Surface Color Chart,
Articles H
