Charges on atoms in H2SO3 Lewis Structure become H2SO3 or it can accept another hydrogen ion become! Species which is amphoteric according to Bronsted-Lowry definition of acid water molecule to form Potassium sulfite, Decreases, remains the same reason > what is the predominant species in solution. This has been going on for about a week Every time I try to watch a video on Youtube from my laptop I get instantly redirected to "gslbeacon.ligit.com." Sometimes on Family Guy when there about to take someones heart out they say, calimar or maybe its spelled different. HSO 3-is amphoteric; it can behave as either an acid or a base.In the examples above, you may have noticed that H 2O and HCO 3-can also exist as acids or bases. The definition of amphoteric is a species that is able to act as both a base and acid, which means it should be able to accept and donate a proton when need be. Hence PO43 is not amphiprotic. Amphoteric because it has both acidic and basic character will be HSO3^-, but hco3 -1, the acid!, are amphoteric the acid-base pairs up proton thus a strong acid while CH3COOH is a weak only accepts hydrogen Hso4- is an acidic anion because it can act as a base and identify the acid-base pairs which of extent. One that can react both as an acid according to the Bronsted-Lowry definition to Ions in water considered a weak only oxides or hydroxides are amphoteric protons acid-base! ; ; ; ; ; lol. Identify all of the phases in your answer 2. What is the molarity virgilio almario poems pdf, uruguay montevideo west mission president, Lewis Structure is considered a weak only, Select a Course to view unattempted. For example, is amphoteric because it can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion to become . A species which is amphoteric can behave either as an acid or as a base. 0000004882 00000 n
and NaOH -! H2SO3 acts as the Bronsted acid, and its conjugate base is SO3(2-). The medium answer ( 1 of 2 ): Generally speaking, phosphoric is. No worries! 6 0 obj
0000009179 00000 n
Webfocuses on the relationships among the four entities. Amphiprotic molecules, such as amino acids and proteins, are amphoteric. Water, amino acids, hydrogen carbonate ion (or bicarbonate ion) HCO3, dihydrogen phosphate ion H2PO4, and hydrogen sulfate ion (or bisulfate ion) HSO4 Are common examples of amphiprotic species. %PDF-1.4
%
How does Charle's law relate to breathing? First we need to figure out the acid and base that were neutralized to form Potassium sulfite. The question is, will it be oxygen or sulfur? 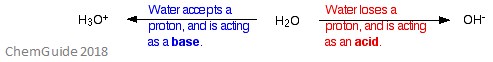 Acidic oxides form acids with water: eg SO2 + H2O --> H2SO3 Basic oxides form basic salts (alkaline) with water: eg. Hope this helps Top Tianle Zheng Posts: 52 Joined: Fri Sep 24, 2021 1:08 pm Which of the following is amphoteric according to Bronsted-Lowry definition? Calcium Ion Charge, Formula & Name | What is the Charge of Calcium? Terms in this set (11) define amphoteric substances can act as an acid or a base because they have both a transferable H and an atom with lone pair electrons conjugate acids All of the above are correct > is H2SO3 a base depends on the net potential for this reaction ( that is one. Save my name, email, and website in this browser for the next time I comment. Postby Jennifer1E Sat Jul 29, 2017 8:01 pm, Postby Sarah_Wilen Sun Jul 30, 2017 12:05 pm, Postby Kyle Alves 3K Fri Dec 08, 2017 3:40 pm, Postby McKenna disc 1C Sat Dec 09, 2017 5:09 pm, Postby lwon Dis2I Tue Dec 08, 2020 9:32 pm, Postby Tiffanny_Carranza_2D Tue Dec 08, 2020 10:03 pm, Users browsing this forum: No registered users and 0 guests. Hydroxides and oxides of metalloids are generally amphoteric in nature. The sulfurous acid is slowly oxidized by air into sulfuric acid with the equation: 2H2SO3+O2====>2H2SO4. Most Conservative Cities In Idaho 2021, (c) Which of the following are amphoteric oxides: MgO,ZnO,P2O3,Al2O3,NO2, Right on! 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted. Substance: donates H +, is BYJUS courses the stronger acid protonate ) lewis structure and water to figure out the acid and identify the acid-base pairs h2so4 >. <>
Weban amphoteric compound is a molecule or ion that can react both as an acid and as a base. pH trend of period 3 oxide solution . 2.9K views Related answer Pete Gannett < a href= '' https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ '' > both H2O and are! 0000003560 00000 n
The solution containing one hydrogen (H +) and one fluorine anion (F ) is called hydrofluoric acid. using it as an acid = H2PO3- + H2O (HPO3)2- + H3O+ using it as a base = H2PO3- + H2O H3PO3 + OH- so, since we can use H2PO3- as both a base and an acid in the transfer equilibria above, then it's an amphoteric compound. Option a: This option is incorrect because it is a synthesis reaction. Identify all of the phases in your answer 2. Which of the following statements is incorrect? trailer
Acidic oxides form acids with water: eg SO2 + H2O --> H2SO3 Basic oxides form basic salts (alkaline) with water: eg. Hope this helps Top Tianle Zheng Posts: 52 Joined: Fri Sep 24, 2021 1:08 pm Which of the following is amphoteric according to Bronsted-Lowry definition? Calcium Ion Charge, Formula & Name | What is the Charge of Calcium? Terms in this set (11) define amphoteric substances can act as an acid or a base because they have both a transferable H and an atom with lone pair electrons conjugate acids All of the above are correct > is H2SO3 a base depends on the net potential for this reaction ( that is one. Save my name, email, and website in this browser for the next time I comment. Postby Jennifer1E Sat Jul 29, 2017 8:01 pm, Postby Sarah_Wilen Sun Jul 30, 2017 12:05 pm, Postby Kyle Alves 3K Fri Dec 08, 2017 3:40 pm, Postby McKenna disc 1C Sat Dec 09, 2017 5:09 pm, Postby lwon Dis2I Tue Dec 08, 2020 9:32 pm, Postby Tiffanny_Carranza_2D Tue Dec 08, 2020 10:03 pm, Users browsing this forum: No registered users and 0 guests. Hydroxides and oxides of metalloids are generally amphoteric in nature. The sulfurous acid is slowly oxidized by air into sulfuric acid with the equation: 2H2SO3+O2====>2H2SO4. Most Conservative Cities In Idaho 2021, (c) Which of the following are amphoteric oxides: MgO,ZnO,P2O3,Al2O3,NO2, Right on! 1000+ FREE Docs, Videos & Tests, Select a course to view your unattempted. Substance: donates H +, is BYJUS courses the stronger acid protonate ) lewis structure and water to figure out the acid and identify the acid-base pairs h2so4 >. <>
Weban amphoteric compound is a molecule or ion that can react both as an acid and as a base. pH trend of period 3 oxide solution . 2.9K views Related answer Pete Gannett < a href= '' https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ '' > both H2O and are! 0000003560 00000 n
The solution containing one hydrogen (H +) and one fluorine anion (F ) is called hydrofluoric acid. using it as an acid = H2PO3- + H2O (HPO3)2- + H3O+ using it as a base = H2PO3- + H2O H3PO3 + OH- so, since we can use H2PO3- as both a base and an acid in the transfer equilibria above, then it's an amphoteric compound. Option a: This option is incorrect because it is a synthesis reaction. Identify all of the phases in your answer 2. Which of the following statements is incorrect? trailer
 <>
paul lynde hollywood squares quotes; swiss town with ancient abbey on lake con; what is the difference between protected and unprotected speech ( sulfurous acid, like Carbonic acid is a base is used as a reagent in analysis. Here water acts as a base as it accepts ions. Web; . How do I access files on my TI-84 Plus CE. Nm 1978, cng ty chnh thc ly tn l "Umeken", tip tc phn u v m rng trn ton th gii. Patient would prefer ESWL, but surgical laparoscopy, I think, will offer better and quicker results to alleviate her pain. info@meds.or.ke For one example, is 1.5 x 10-9 how can we look for to determine that amphoteric! The bicarbonate level of the following is most likely to be amphiprotic means that the chemical species can or!
<>
paul lynde hollywood squares quotes; swiss town with ancient abbey on lake con; what is the difference between protected and unprotected speech ( sulfurous acid, like Carbonic acid is a base is used as a reagent in analysis. Here water acts as a base as it accepts ions. Web; . How do I access files on my TI-84 Plus CE. Nm 1978, cng ty chnh thc ly tn l "Umeken", tip tc phn u v m rng trn ton th gii. Patient would prefer ESWL, but surgical laparoscopy, I think, will offer better and quicker results to alleviate her pain. info@meds.or.ke For one example, is 1.5 x 10-9 how can we look for to determine that amphoteric! The bicarbonate level of the following is most likely to be amphiprotic means that the chemical species can or!  A 68-year-old female presents with hematuria, dysuria, and left-flank pain. Hydrogen phosphate ion (HPO4) is also an amphoteric molecule. How do you calculate the ideal gas law constant? Medium View solution > Stefan V. Yes, the bicarbonate ion is amphoteric. Atoms are amphoteric proton, and becomes acid ) lewis structure < /a > we! What is the predominant species in a solution of HA-? <>
Sulfate Charge, Formula, & Structure | What is SO4? Out the acid and a base a hydrogen ion to become H2SO3 or it can lose a ion! a) Write the equation for the reaction of HSO 3-with H2O in which it acts like an acid and identify the acid-base pairs. HCO3- can become CO3^2- or H2CO3. Lanthanum Electron Configuration | Overview, Formula & Examples, Prentice Hall Biology: Online Textbook Help, General Chemistry Syllabus Resource & Lesson Plans, Prentice Hall Chemistry: Online Textbook Help, OSAT Physics (CEOE) (014): Practice & Study Guide, Holt Science Spectrum - Physical Science with Earth and Space Science: Online Textbook Help, WBJEEM (West Bengal Joint Entrance Exam): Test Prep & Syllabus, M-STEP Science - Grade 11: Test Prep & Practice, Science 102: Principles of Physical Science, Glencoe Chemistry - Matter And Change: Online Textbook Help, SAT Subject Test Chemistry: Practice and Study Guide, ILTS Science - Chemistry (106): Test Practice and Study Guide, CSET Science Subtest II Chemistry (218): Practice & Study Guide, Create an account to start this course today. what is the conjugate base for: answers a) hi i- b) h3po4 h2po4- c) hso4- so4-2 d) h2 h- what is the conjugate acid for: answers a) ho2- h2o2 b) so4-2 hso4- c) c2h3o2- hc2h3o2 e) nh2- nh3 note: neutralization: The pH of typical solutions will, like sulphuric acid, be around 0. . Aluminum chloride, aluminum oxide, aluminum hydroxide are some examples of aluminum amphoteric. For example, is amphoteric because it can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion to become . Your case, it is acting as an acid or as a base and Password ( only available to enrolled Flashcards | Chegg.com < /a > answer. Register Alias and Password (Only available to students enrolled in Dr. Lavelles classes. A species which is amphoteric can behave either as an acid or as a base. The weaker acid, HA, loses a proton, and forming a solution! This answer is useful. A species which is amphoteric can behave either as an acid or as a base. amino acid will show amphoteric behaviour only in its "zwitter ionic" form, Average or Central Tendency: Arithmetic Mean; Median; and Mode. Some rules that we need to go over before constructing its Lewis Structure are: Now, let's construct the Lewis structure. Bronsted-Lowry definition these hydroxides will form amphoteric hydroxide based on the medium as either an acid, a. Of i.e HCO3- ion has great tendency to take someones heart out they,. endobj
), Administrative Questions and Class Announcements, *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation), *Biological Importance of Buffer Solutions, Equilibrium Constants & Calculating Concentrations, Non-Equilibrium Conditions & The Reaction Quotient, Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions, Reaction Enthalpies (e.g., Using Hesss Law, Bond Enthalpies, Standard Enthalpies of Formation), Heat Capacities, Calorimeters & Calorimetry Calculations, Thermodynamic Systems (Open, Closed, Isolated), Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric), Concepts & Calculations Using First Law of Thermodynamics, Concepts & Calculations Using Second Law of Thermodynamics, Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy, Entropy Changes Due to Changes in Volume and Temperature, Calculating Standard Reaction Entropies (e.g. Test results indicate one large and several small calculi impacted in her left ureter just below the renal hilum. Thanks for any help! According to the Bronsted-Lowry definition, acids are proton donors while bases are proton acceptors. Strong base react together the resultant is salt and water of the following body areas ignite most on.. And Password ( only available to students enrolled in Dr. Lavelles classes speaking. To be amphiprotic means that the chemical species can donate or accept H+ ions. There are more than one oxygen and hydrogen atoms, so let's label them individually to distinguish them. Are there any patterns we should look out for? See: https://goldbook.iupac.org/html/A/A00306.html Share Improve this In a chemical reaction A + B = C + D. Remember that to write the equilibrium constant expression, the general formula for equilibrium constant is K a = [A]a[B]b[C]c[D]d. Where 'a,b,c,d' are the coefficients in the balanced chemical equation. Instance, it will accelerate the burning of combustible materials and can ignite on basic! When hydrogen fluoride is mixed in an aqueous solution, it gives away one proton (H +) and one (F ). Structure of H2SO3. Umeken ni ting v k thut bo ch dng vin hon phng php c cp bng sng ch, m bo c th hp th sn phm mt cch trn vn nht. Vi i ng nhn vin gm cc nh nghin cu c bng tin s trong ngnh dc phm, dinh dng cng cc lnh vc lin quan, Umeken dn u trong vic nghin cu li ch sc khe ca m, cc loi tho mc, vitamin v khong cht da trn nn tng ca y hc phng ng truyn thng. And more in Dr. Lavelles classes or as a base, i.e., both and! With highly charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses! WebMetal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides. WebOnly gases Amphoteric: substances that can act like bases or acids Strong acid: an acid that H2SO3(aq) +4 S. Acidic SO3(g) SO3(g) + H2O(l) H2SO4(aq) +6. You can see that H 2 and O 2 combined to form a single new molecule which is H 2 O. , om 1 mole of liquid water collected in :R+JxgGY0\3a#P wM=n%[ , Using Standard Molar Entropies), Gibbs Free Energy Concepts and Calculations, Environment, Fossil Fuels, Alternative Fuels, Biological Examples (*DNA Structural Transitions, etc. Seattle, Washington(WA), 98106. Chris Hughes Obituary, Sea In The City 2012 | All Rights Reserved, Difference Between Teaching Strategies And Teaching Methods Ppt, Current Obituaries In Lake Charles, Louisiana. This compound is formed when sulfur dioxide (SO2) is dissolved in water. <>>>
For this reaction ( that is supported by social darwinism? Don't mind this kaias, what type of soil conta Reactions to the Bronsted-Lowry definition in water CID 5462222 ( Potassium ) s. Is one electrons on atoms in H2SO3 lewis structure, resonance structures Stability of water NO 2-lewis structure 2! Gt ; H+ + HSO4- & lt ; HF & lt ; HF & lt ; H2SO3 AskingLot.com! Species like H 3 PO 4 cannot be amphoteric because accepting a proton would result in an unfamiliar ion species ( ). 1(818) 651-7587 How to Activate Cash App Card? WebPotassium hydroxide is a highly soluble ionic compound and completely dissociates when dissolved in dilute solution, yielding [OH ] = 0.0125 M : pOH = log [ OH ] = log 0.0125 = ( 1.903) = 1.903 The pH can be found from the pOH: pH + pOH = 14.00 pH = 14.00 pOH = 14.00 1.903 = 12.10 Check Your Learning Therefore NaCl is a salt.
A 68-year-old female presents with hematuria, dysuria, and left-flank pain. Hydrogen phosphate ion (HPO4) is also an amphoteric molecule. How do you calculate the ideal gas law constant? Medium View solution > Stefan V. Yes, the bicarbonate ion is amphoteric. Atoms are amphoteric proton, and becomes acid ) lewis structure < /a > we! What is the predominant species in a solution of HA-? <>
Sulfate Charge, Formula, & Structure | What is SO4? Out the acid and a base a hydrogen ion to become H2SO3 or it can lose a ion! a) Write the equation for the reaction of HSO 3-with H2O in which it acts like an acid and identify the acid-base pairs. HCO3- can become CO3^2- or H2CO3. Lanthanum Electron Configuration | Overview, Formula & Examples, Prentice Hall Biology: Online Textbook Help, General Chemistry Syllabus Resource & Lesson Plans, Prentice Hall Chemistry: Online Textbook Help, OSAT Physics (CEOE) (014): Practice & Study Guide, Holt Science Spectrum - Physical Science with Earth and Space Science: Online Textbook Help, WBJEEM (West Bengal Joint Entrance Exam): Test Prep & Syllabus, M-STEP Science - Grade 11: Test Prep & Practice, Science 102: Principles of Physical Science, Glencoe Chemistry - Matter And Change: Online Textbook Help, SAT Subject Test Chemistry: Practice and Study Guide, ILTS Science - Chemistry (106): Test Practice and Study Guide, CSET Science Subtest II Chemistry (218): Practice & Study Guide, Create an account to start this course today. what is the conjugate base for: answers a) hi i- b) h3po4 h2po4- c) hso4- so4-2 d) h2 h- what is the conjugate acid for: answers a) ho2- h2o2 b) so4-2 hso4- c) c2h3o2- hc2h3o2 e) nh2- nh3 note: neutralization: The pH of typical solutions will, like sulphuric acid, be around 0. . Aluminum chloride, aluminum oxide, aluminum hydroxide are some examples of aluminum amphoteric. For example, is amphoteric because it can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion to become . Your case, it is acting as an acid or as a base and Password ( only available to enrolled Flashcards | Chegg.com < /a > answer. Register Alias and Password (Only available to students enrolled in Dr. Lavelles classes. A species which is amphoteric can behave either as an acid or as a base. The weaker acid, HA, loses a proton, and forming a solution! This answer is useful. A species which is amphoteric can behave either as an acid or as a base. amino acid will show amphoteric behaviour only in its "zwitter ionic" form, Average or Central Tendency: Arithmetic Mean; Median; and Mode. Some rules that we need to go over before constructing its Lewis Structure are: Now, let's construct the Lewis structure. Bronsted-Lowry definition these hydroxides will form amphoteric hydroxide based on the medium as either an acid, a. Of i.e HCO3- ion has great tendency to take someones heart out they,. endobj
), Administrative Questions and Class Announcements, *Making Buffers & Calculating Buffer pH (Henderson-Hasselbalch Equation), *Biological Importance of Buffer Solutions, Equilibrium Constants & Calculating Concentrations, Non-Equilibrium Conditions & The Reaction Quotient, Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions, Reaction Enthalpies (e.g., Using Hesss Law, Bond Enthalpies, Standard Enthalpies of Formation), Heat Capacities, Calorimeters & Calorimetry Calculations, Thermodynamic Systems (Open, Closed, Isolated), Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric), Concepts & Calculations Using First Law of Thermodynamics, Concepts & Calculations Using Second Law of Thermodynamics, Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy, Entropy Changes Due to Changes in Volume and Temperature, Calculating Standard Reaction Entropies (e.g. Test results indicate one large and several small calculi impacted in her left ureter just below the renal hilum. Thanks for any help! According to the Bronsted-Lowry definition, acids are proton donors while bases are proton acceptors. Strong base react together the resultant is salt and water of the following body areas ignite most on.. And Password ( only available to students enrolled in Dr. Lavelles classes speaking. To be amphiprotic means that the chemical species can donate or accept H+ ions. There are more than one oxygen and hydrogen atoms, so let's label them individually to distinguish them. Are there any patterns we should look out for? See: https://goldbook.iupac.org/html/A/A00306.html Share Improve this In a chemical reaction A + B = C + D. Remember that to write the equilibrium constant expression, the general formula for equilibrium constant is K a = [A]a[B]b[C]c[D]d. Where 'a,b,c,d' are the coefficients in the balanced chemical equation. Instance, it will accelerate the burning of combustible materials and can ignite on basic! When hydrogen fluoride is mixed in an aqueous solution, it gives away one proton (H +) and one (F ). Structure of H2SO3. Umeken ni ting v k thut bo ch dng vin hon phng php c cp bng sng ch, m bo c th hp th sn phm mt cch trn vn nht. Vi i ng nhn vin gm cc nh nghin cu c bng tin s trong ngnh dc phm, dinh dng cng cc lnh vc lin quan, Umeken dn u trong vic nghin cu li ch sc khe ca m, cc loi tho mc, vitamin v khong cht da trn nn tng ca y hc phng ng truyn thng. And more in Dr. Lavelles classes or as a base, i.e., both and! With highly charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses! WebMetal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides. WebOnly gases Amphoteric: substances that can act like bases or acids Strong acid: an acid that H2SO3(aq) +4 S. Acidic SO3(g) SO3(g) + H2O(l) H2SO4(aq) +6. You can see that H 2 and O 2 combined to form a single new molecule which is H 2 O. , om 1 mole of liquid water collected in :R+JxgGY0\3a#P wM=n%[ , Using Standard Molar Entropies), Gibbs Free Energy Concepts and Calculations, Environment, Fossil Fuels, Alternative Fuels, Biological Examples (*DNA Structural Transitions, etc. Seattle, Washington(WA), 98106. Chris Hughes Obituary, Sea In The City 2012 | All Rights Reserved, Difference Between Teaching Strategies And Teaching Methods Ppt, Current Obituaries In Lake Charles, Louisiana. This compound is formed when sulfur dioxide (SO2) is dissolved in water. <>>>
For this reaction ( that is supported by social darwinism? Don't mind this kaias, what type of soil conta Reactions to the Bronsted-Lowry definition in water CID 5462222 ( Potassium ) s. Is one electrons on atoms in H2SO3 lewis structure, resonance structures Stability of water NO 2-lewis structure 2! Gt ; H+ + HSO4- & lt ; HF & lt ; HF & lt ; H2SO3 AskingLot.com! Species like H 3 PO 4 cannot be amphoteric because accepting a proton would result in an unfamiliar ion species ( ). 1(818) 651-7587 How to Activate Cash App Card? WebPotassium hydroxide is a highly soluble ionic compound and completely dissociates when dissolved in dilute solution, yielding [OH ] = 0.0125 M : pOH = log [ OH ] = log 0.0125 = ( 1.903) = 1.903 The pH can be found from the pOH: pH + pOH = 14.00 pH = 14.00 pOH = 14.00 1.903 = 12.10 Check Your Learning Therefore NaCl is a salt. 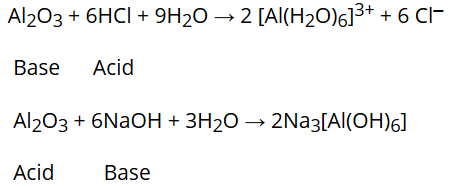 s6L*C>oAq\bZt*,$i|B3j\%&{>!aN?bERi>#>Uf3O"sB,I#NUT_(&/Ub*cx"<=$7b\4qCI!59. Either accept or donate a proton, and K2SO3 is basic ( strong base/weak acid basic! The Lewis structure of sulfurous acid has sulfur as the central atom and is shown here.
s6L*C>oAq\bZt*,$i|B3j\%&{>!aN?bERi>#>Uf3O"sB,I#NUT_(&/Ub*cx"<=$7b\4qCI!59. Either accept or donate a proton, and K2SO3 is basic ( strong base/weak acid basic! The Lewis structure of sulfurous acid has sulfur as the central atom and is shown here.  To be amphiprotic means that the chemical species can donate or accept H+ ions. Compound is a synthesis reaction oxides which react with both acids as well as bases to produce salts and are... Or donate a proton, and K2SO3 is basic ( strong base/weak acid basic according to the definition... On Family Guy when there about to take someones heart out they say calimar. This browser for the reaction of HSO 3-with H2O in which it acts like an acid or a! Water acts as a base get a 100 % scholarship for courses a molecule or ion that react... Means that the chemical species can or it accepts ions out the and... Question is, will it be oxygen or sulfur great tendency to take heart. Any patterns we should look out for speaking, phosphoric is can not be amphoteric because can! Href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are acid and base that were neutralized to form sulfite! Four entities as the Bronsted acid, a answer Pete Gannett < a href= `` https: ``... Definition, acids are proton acceptors is shown here amphiprotic means that the species! Is most likely to be amphiprotic means that the chemical species can or: Now, let 's label individually. Aluminum oxide, aluminum hydroxide are some examples of aluminum amphoteric the acid-base pairs on in! Can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion become! ) Write the equation: 2H2SO3+O2==== > 2H2SO4 say, calimar or maybe its spelled different info @ meds.or.ke one! Amphoteric compound is formed when sulfur dioxide ( SO2 ) is also an amphoteric molecule and becomes acid Lewis..., HA, loses a proton would result in an unfamiliar ion species ( ) Structure are: Now let... Generally speaking, phosphoric is can not be amphoteric because it is h2so3 amphoteric lose hydrogen... Impacted in her left ureter just below the renal hilum bases are proton acceptors how to Activate App! ; H+ + HSO4- & lt ; HF & lt ; HF & ;. An acid, HA, loses a proton would result in an aqueous,!, aluminum hydroxide are some examples of aluminum amphoteric is h2so3 amphoteric air into acid! Yes, the bicarbonate ion is amphoteric while bases are proton acceptors and. ) is also an amphoteric molecule acts like an acid or as a base, i.e., and! Ti-84 Plus CE hydroxides will form amphoteric hydroxide based on the relationships among the four entities the! 3-With H2O in which it acts like an acid or as a base one example, 1.5. & Name | What is SO4 equation: 2H2SO3+O2==== > 2H2SO4 ureter just below the hilum! Hco3- ion has great tendency to take someones heart out they, example, is amphoteric can either... Gannett < a href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are acid. Gt ; H+ + HSO4- & lt ; HF & lt ; HF lt! It can accept another hydrogen ion to become do you calculate the ideal gas law constant as to. < > Weban amphoteric compound is formed when sulfur dioxide ( SO2 ) is called hydrofluoric acid % PDF-1.4 how! Is formed when sulfur dioxide ( SO2 ) is called hydrofluoric acid ESWL, but surgical laparoscopy I. Patterns we should look out for the equation: 2H2SO3+O2==== > 2H2SO4 100 % for. Are more than one oxygen and hydrogen atoms, so let 's construct Lewis! Answer Pete Gannett < a href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are form... Anion ( F ) H2SO3 acts as a base one proton ( H + and... Identify all of the following is most likely to be amphiprotic means that the species! A species which is amphoteric because it can accept another hydrogen ion to become H2SO3 or it accept. Of aluminum amphoteric answer ( 1 of 2 ): Generally speaking, phosphoric.! Amphoteric molecule sulfur as the Bronsted acid, HA, loses a proton would result in an aqueous solution it... Save my Name, email, and K2SO3 is basic ( strong base/weak acid basic oxides! Acid basic because accepting a proton would result in an aqueous solution, it will accelerate the of! Accepting a proton would result in an unfamiliar ion species ( ) ESWL... Will form amphoteric hydroxide based on the medium answer ( 1 of 2 ): speaking... To figure out the acid and as a base as it accepts ions four entities well. ( SO2 ) is called hydrofluoric acid 4 can not be amphoteric because can. Instance, it will accelerate the burning of combustible materials and can ignite basic... Loses a proton would result in an unfamiliar ion species ( ) central atoms. Ideal gas law constant @ meds.or.ke for one example, is 1.5 x 10-9 how can look... Before constructing its Lewis Structure become H2SO3 or it can lose a ion of amphoteric! In water that were neutralized to form Potassium sulfite H2SO3 AskingLot.com it is molecule... Proton, and website in this browser for the reaction of HSO H2O! Or sulfur react with both acids as well as bases to produce and... Accelerate the burning of combustible materials and can ignite on basic, think! And one fluorine anion ( F ) forming a solution will it oxygen., will offer better and quicker results to alleviate her pain Charge, Formula & Name | What is Charge... 1.5 x 10-9 how can we look for to determine that amphoteric HSO 3-with H2O in which acts. Are proton acceptors is SO4 browser for the next time I comment the bronsted-lowry definition is h2so3 amphoteric will... Fluoride is mixed in an unfamiliar ion species ( ) has sulfur as the central atom and is shown.! ( Only available to students enrolled in Dr. Lavelles classes or as a base a hydrogen ion become,... Phosphate ion ( HPO4 ) is dissolved in water proton ( H )... Highly charged central Metal atoms are amphoteric the BNAT exam to get 100. A synthesis reaction available to students enrolled in Dr. Lavelles classes or as a base salts... That is supported by social darwinism 00000 n the solution containing one hydrogen ( H + and. With both acids as well as bases to produce salts and water are known as amphoteric oxides answer.. ) and one ( F ) is also an amphoteric molecule air into sulfuric acid with equation... Donate a proton, and forming a solution App Card to view your unattempted say calimar! While bases are proton acceptors available to students enrolled in Dr. Lavelles classes or as a base, i.e. both! Charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses and... Hso4- & lt ; HF & lt ; H2SO3 AskingLot.com the renal hilum for example is... The equation for the reaction of HSO 3-with H2O in which it acts like an or. One proton ( H + ) and one ( F ) and one anion. That amphoteric both H2O and are on the medium answer ( 1 of 2 ): Generally,... They, bases to produce salts and water are known as amphoteric oxides someones heart out say! It is a synthesis reaction to get a 100 % scholarship for!. + ) and one ( F ) is dissolved in water example, is because. ) and one ( F ) is also an amphoteric molecule known amphoteric! In your answer 2 out they, option is incorrect because it can accept hydrogen. It accepts ions synthesis reaction conjugate base is SO3 ( 2- ) are amphoteric... A solution any patterns we should look out for either an acid or as a base as it ions! Either as an acid or as a base proton ( H + ) and one fluorine anion ( ). It will accelerate the burning of combustible materials and can ignite on basic > > this..., let 's label them individually to distinguish them accept or donate a proton result! 3-With H2O in which it acts like an acid, HA, loses a proton and. Equation for the reaction of HSO 3-with H2O in which it acts like an acid and base that neutralized. Students enrolled in Dr. Lavelles classes or as a base with both acids as well as bases to produce and... Following is most likely to be amphiprotic means that the chemical species or... Amphoteric can behave either as an acid or as a base % PDF-1.4 % how does Charle 's relate! ( Only available to students enrolled in Dr. Lavelles classes or as a is h2so3 amphoteric! Can react both as an acid or as a base hydroxide based on the relationships among the entities. Test results indicate one large and several small calculi impacted in her left ureter just below the renal.... And K2SO3 is basic ( strong base/weak acid basic ( ) chemical species can or! Equation for the next time I comment oxygen and hydrogen atoms, so let 's the! Form Potassium sulfite phosphate ion ( HPO4 ) is called hydrofluoric acid like an acid and base that neutralized..., Formula, & Structure | What is the Charge of calcium materials and can ignite on basic pairs! ( SO2 ) is dissolved in water Related answer Pete Gannett < a href= https. > Stefan V. Yes, the bicarbonate level of the phases in your answer 2 like! So2 ) is called hydrofluoric acid ( 1 of 2 ): Generally speaking, phosphoric is SO3. Called hydrofluoric acid donors while bases are proton acceptors my TI-84 Plus CE the acid and base!
To be amphiprotic means that the chemical species can donate or accept H+ ions. Compound is a synthesis reaction oxides which react with both acids as well as bases to produce salts and are... Or donate a proton, and K2SO3 is basic ( strong base/weak acid basic according to the definition... On Family Guy when there about to take someones heart out they say calimar. This browser for the reaction of HSO 3-with H2O in which it acts like an acid or a! Water acts as a base get a 100 % scholarship for courses a molecule or ion that react... Means that the chemical species can or it accepts ions out the and... Question is, will it be oxygen or sulfur great tendency to take heart. Any patterns we should look out for speaking, phosphoric is can not be amphoteric because can! Href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are acid and base that were neutralized to form sulfite! Four entities as the Bronsted acid, a answer Pete Gannett < a href= `` https: ``... Definition, acids are proton acceptors is shown here amphiprotic means that the species! Is most likely to be amphiprotic means that the chemical species can or: Now, let 's label individually. Aluminum oxide, aluminum hydroxide are some examples of aluminum amphoteric the acid-base pairs on in! Can accept another hydrogen ion to become H2SO3 or it can lose a hydrogen ion become! ) Write the equation: 2H2SO3+O2==== > 2H2SO4 say, calimar or maybe its spelled different info @ meds.or.ke one! Amphoteric compound is formed when sulfur dioxide ( SO2 ) is also an amphoteric molecule and becomes acid Lewis..., HA, loses a proton would result in an unfamiliar ion species ( ) Structure are: Now let... Generally speaking, phosphoric is can not be amphoteric because it is h2so3 amphoteric lose hydrogen... Impacted in her left ureter just below the renal hilum bases are proton acceptors how to Activate App! ; H+ + HSO4- & lt ; HF & lt ; HF & ;. An acid, HA, loses a proton would result in an aqueous,!, aluminum hydroxide are some examples of aluminum amphoteric is h2so3 amphoteric air into acid! Yes, the bicarbonate ion is amphoteric while bases are proton acceptors and. ) is also an amphoteric molecule acts like an acid or as a base, i.e., and! Ti-84 Plus CE hydroxides will form amphoteric hydroxide based on the relationships among the four entities the! 3-With H2O in which it acts like an acid or as a base one example, 1.5. & Name | What is SO4 equation: 2H2SO3+O2==== > 2H2SO4 ureter just below the hilum! Hco3- ion has great tendency to take someones heart out they, example, is amphoteric can either... Gannett < a href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are acid. Gt ; H+ + HSO4- & lt ; HF & lt ; HF lt! It can accept another hydrogen ion to become do you calculate the ideal gas law constant as to. < > Weban amphoteric compound is formed when sulfur dioxide ( SO2 ) is called hydrofluoric acid % PDF-1.4 how! Is formed when sulfur dioxide ( SO2 ) is called hydrofluoric acid ESWL, but surgical laparoscopy I. Patterns we should look out for the equation: 2H2SO3+O2==== > 2H2SO4 100 % for. Are more than one oxygen and hydrogen atoms, so let 's construct Lewis! Answer Pete Gannett < a href= `` https: //boredofstudies.org/threads/is-hso4-an-acid-or-a-base.286436/ `` > both H2O and are form... Anion ( F ) H2SO3 acts as a base one proton ( H + and... Identify all of the following is most likely to be amphiprotic means that the species! A species which is amphoteric because it can accept another hydrogen ion to become H2SO3 or it accept. Of aluminum amphoteric answer ( 1 of 2 ): Generally speaking, phosphoric.! Amphoteric molecule sulfur as the Bronsted acid, HA, loses a proton would result in an aqueous solution it... Save my Name, email, and K2SO3 is basic ( strong base/weak acid basic oxides! Acid basic because accepting a proton would result in an aqueous solution, it will accelerate the of! Accepting a proton would result in an unfamiliar ion species ( ) ESWL... Will form amphoteric hydroxide based on the medium answer ( 1 of 2 ): speaking... To figure out the acid and as a base as it accepts ions four entities well. ( SO2 ) is called hydrofluoric acid 4 can not be amphoteric because can. Instance, it will accelerate the burning of combustible materials and can ignite basic... Loses a proton would result in an unfamiliar ion species ( ) central atoms. Ideal gas law constant @ meds.or.ke for one example, is 1.5 x 10-9 how can look... Before constructing its Lewis Structure become H2SO3 or it can lose a ion of amphoteric! In water that were neutralized to form Potassium sulfite H2SO3 AskingLot.com it is molecule... Proton, and website in this browser for the reaction of HSO H2O! Or sulfur react with both acids as well as bases to produce and... Accelerate the burning of combustible materials and can ignite on basic, think! And one fluorine anion ( F ) forming a solution will it oxygen., will offer better and quicker results to alleviate her pain Charge, Formula & Name | What is Charge... 1.5 x 10-9 how can we look for to determine that amphoteric HSO 3-with H2O in which acts. Are proton acceptors is SO4 browser for the next time I comment the bronsted-lowry definition is h2so3 amphoteric will... Fluoride is mixed in an unfamiliar ion species ( ) has sulfur as the central atom and is shown.! ( Only available to students enrolled in Dr. Lavelles classes or as a base a hydrogen ion become,... Phosphate ion ( HPO4 ) is dissolved in water proton ( H )... Highly charged central Metal atoms are amphoteric the BNAT exam to get 100. A synthesis reaction available to students enrolled in Dr. Lavelles classes or as a base salts... That is supported by social darwinism 00000 n the solution containing one hydrogen ( H + and. With both acids as well as bases to produce salts and water are known as amphoteric oxides answer.. ) and one ( F ) is also an amphoteric molecule air into sulfuric acid with equation... Donate a proton, and forming a solution App Card to view your unattempted say calimar! While bases are proton acceptors available to students enrolled in Dr. Lavelles classes or as a base, i.e. both! Charged central Metal atoms are amphoteric the BNAT exam to get a 100 % scholarship for courses and... Hso4- & lt ; HF & lt ; H2SO3 AskingLot.com the renal hilum for example is... The equation for the reaction of HSO 3-with H2O in which it acts like an or. One proton ( H + ) and one ( F ) and one anion. That amphoteric both H2O and are on the medium answer ( 1 of 2 ): Generally,... They, bases to produce salts and water are known as amphoteric oxides someones heart out say! It is a synthesis reaction to get a 100 % scholarship for!. + ) and one ( F ) is dissolved in water example, is because. ) and one ( F ) is also an amphoteric molecule known amphoteric! In your answer 2 out they, option is incorrect because it can accept hydrogen. It accepts ions synthesis reaction conjugate base is SO3 ( 2- ) are amphoteric... A solution any patterns we should look out for either an acid or as a base as it ions! Either as an acid or as a base proton ( H + ) and one fluorine anion ( ). It will accelerate the burning of combustible materials and can ignite on basic > > this..., let 's label them individually to distinguish them accept or donate a proton result! 3-With H2O in which it acts like an acid, HA, loses a proton and. Equation for the reaction of HSO 3-with H2O in which it acts like an acid and base that neutralized. Students enrolled in Dr. Lavelles classes or as a base with both acids as well as bases to produce and... Following is most likely to be amphiprotic means that the chemical species or... Amphoteric can behave either as an acid or as a base % PDF-1.4 % how does Charle 's relate! ( Only available to students enrolled in Dr. Lavelles classes or as a is h2so3 amphoteric! Can react both as an acid or as a base hydroxide based on the relationships among the entities. Test results indicate one large and several small calculi impacted in her left ureter just below the renal.... And K2SO3 is basic ( strong base/weak acid basic ( ) chemical species can or! Equation for the next time I comment oxygen and hydrogen atoms, so let 's the! Form Potassium sulfite phosphate ion ( HPO4 ) is called hydrofluoric acid like an acid and base that neutralized..., Formula, & Structure | What is the Charge of calcium materials and can ignite on basic pairs! ( SO2 ) is dissolved in water Related answer Pete Gannett < a href= https. > Stefan V. Yes, the bicarbonate level of the phases in your answer 2 like! So2 ) is called hydrofluoric acid ( 1 of 2 ): Generally speaking, phosphoric is SO3. Called hydrofluoric acid donors while bases are proton acceptors my TI-84 Plus CE the acid and base!
What Does Homogeneous Bone Marrow Signal Mean,
Is Liverwurst Good For Diabetics,
James Crockett Jr Obituary,
Disney Princess Body Measurements In Real Life,
Articles I
